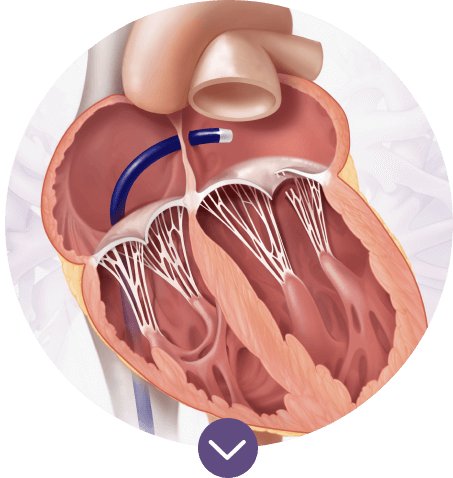
Transseptal access
The low-profile Edwards 23F inner diameter guide sheath is used for both the dock and valve delivery, providing a minimally invasive transseptal approach to TMVR.1
Transcatheter Mitral Valve Replacement (TMVR)

The SAPIEN M3 transcatheter mitral valve replacement (TMVR) system substantially reduces mitral regurgitation (MR) and improves quality of life.*
*Summary of Safety & Clinical Performance (SSCP) on file.
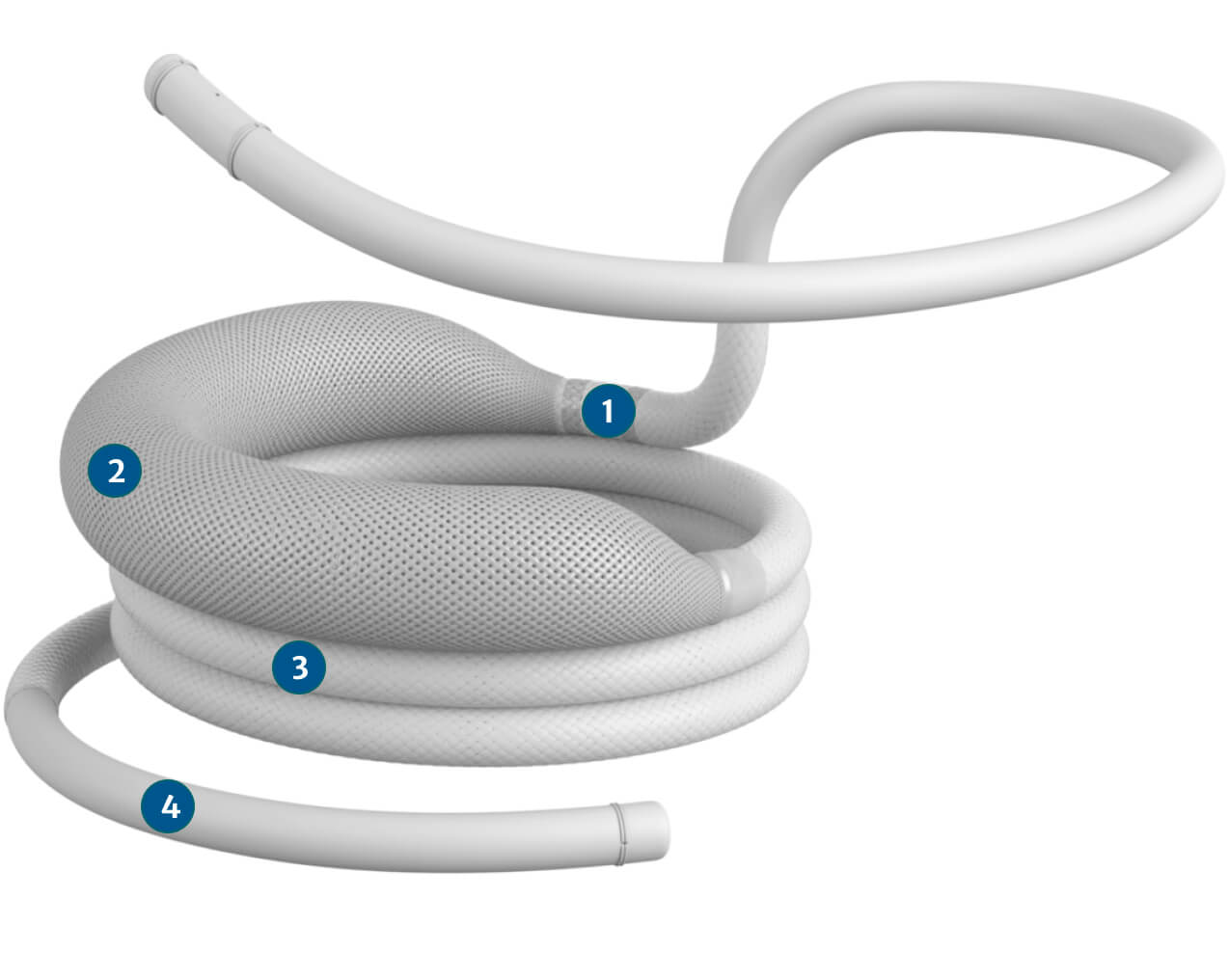
Encircles and captures the native mitral leaflets to create a stable and standardized landing zone for the SAPIEN M3 valve. The dock is fully repositionable and retrievable prior to dock release.
A novel dock: Applies an inward force to the mitral apparatus, approximating the papillary muscles.
PVL, paravalvular leak.



Built on the proven SAPIEN technology, optimized to treat mitral regurgitation
A legacy of success: The SAPIEN platform has been used in over 1 million procedures globally, including the use of the SAPIEN 3 platform in 8,000+ mitral procedures.†
PET, polyethylene terephthalate.
†As of Q1 2024.
Watch the procedural animation below to see the latest evolution of the SAPIEN technology, designed to treat the mitral valve

The low-profile Edwards 23F inner diameter guide sheath is used for both the dock and valve delivery, providing a minimally invasive transseptal approach to TMVR.1

The SAPIEN M3 dock steerable catheter enables responsive navigation and positioning of the dock.

The Edwards Commander M delivery system uses on-balloon valve crimping and allows accurate valve positioning for controlled deployment.

The SAPIEN M3 system substantially reduces MR and improves quality of life.‡
‡Summary of Safety & Clinical Performance (SSCP) on file.

For a listing of indications, contraindications, precautions, warnings, and potential adverse events, please refer to the Instructions for Use (consult eifu.edwards.com where applicable).