
Choosing an aortic valve that does not compromise your patient’s quality of life or future treatment approaches is crucial
Get closer to innovation.

With its enhanced anti-calcification procedure and VFit technology, the INSPIRIS RESILIA aortic valve has the potential to offer your patients a longer-lasting valve that does not limit their future treatment options.1
The first valve choice dictates the lifetime management of aortic valve disease.2 Mechanical valves offer durability but the continuous anticoagulation with warfarin complicates management and is associated with severe risk of bleeding.3,4 As a result, patients are increasingly turning to tissue valves.3,5 With life expectancy on the rise,6 patients need longer lasting valves that also won’t limit their future treatment options.
Enhanced anti-calcification technology1,7–11
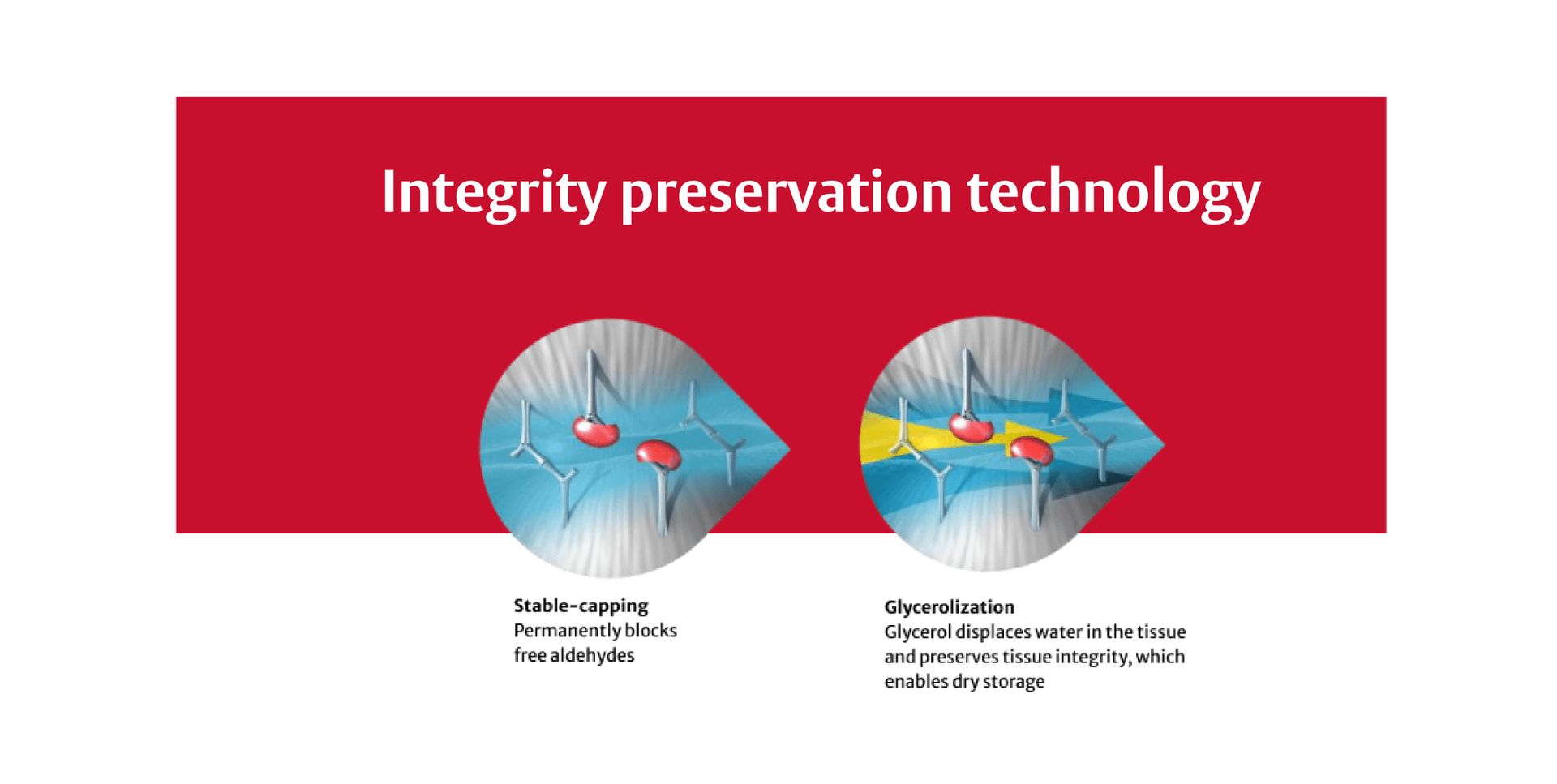
RESILIA tissue is bovine pericardial tissue treated with a special integrity preservation technology that mitigates free aldehydes, potentially allowing the valve to last longer.*1,7–11
In other glutaraldehyde-fixed tissues, the treatment chemicals are able to diffuse out of the tissue over time. This means those tissues become increasingly susceptible to calcification.11,12
The INSPIRIS RESILIA aortic valve is made of RESILIA tissue which undergoes a proprietary process that mitigates free aldehydes.1,7–9





*Based on bench data.
Irrespective of the choice of bioprosthesis, some patients will require a second intervention. The INSPIRIS RESILIA aortic valve has been designed to enable flexibility of future treatment options for both surgeons and patients.1
Other tissue valves may require valve fracture as part of a valve-in-valve (ViV) procedure, but it has been associated with higher odds of in-hospital mortality (odds ratio 2.51, P<0.01) and life-threatening bleeding (odds ratio 2.55, P<0.01).13 Unlike other valves, the INSPIRIS RESILIA aortic valve is specifically designed to deliver a controlled and predictable expansion during potential future ViV deployment.†14–16

†Based on bench data.
‡Refer to device instructions for important warnings related to VFit technology. These features have not been observed in clinical studies to establish the safety and effectiveness of the model 11500A for use in ViV procedures. VFit technology is available on sizes 19–25 mm.1
FOR MODEL 11500A SIZES 19–25 MM ONLY.
WARNING: DO NOT PERFORM STAND-ALONE BALLOON AORTIC VALVULOPLASTY PROCEDURES ON THIS VALVE FOR THE SIZES 19 – 25 mm. Although the valve will maintain a stable diameter at implant and during intracardiac conditions, the diameter of this valve will expand if radial force is applied, such as during a balloon aortic valvuloplasty. This may expand the valve causing aortic incompetence, coronary embolism or annular rupture.
The expansion zone is activated by applied radial force.

WARNING: Valve-in-valve sizing in the INSPIRIS RESILIA aortic valve has only been tested with specific Edwards transcatheter heart valves. Use of other transcatheter valves may result in embolization of transcatheter devices anchored within or result in annular rupture. Refer to device instructions for use for full prescribing and safety information.
Sign up now and don't miss any updates about our surgical therapies.

SAVR, surgical aortic valve replacement; ViV, valve-in-valve.
No clinical data are available that evaluate the long-term impact of RESILIA tissue in patients. Additional clinical data for up to 10 years of follow-up are being collected to monitor the long-term safety and performance of RESILIA tissue.
Refer to device instructions for use for important warnings related to VFit technology. These features have not been observed in clinical studies to establish the safety and effectiveness of the model 11500A for use in valve-in-valve procedures. VFit technology is available on sizes 19–25 mm.
For a listing of indications, contraindications, precautions, warnings, and potential adverse events, please refer to the Instructions for Use (consult eifu.edwards.com where applicable).