MITRIS RESILIA Mitral Valve

Model 11400M
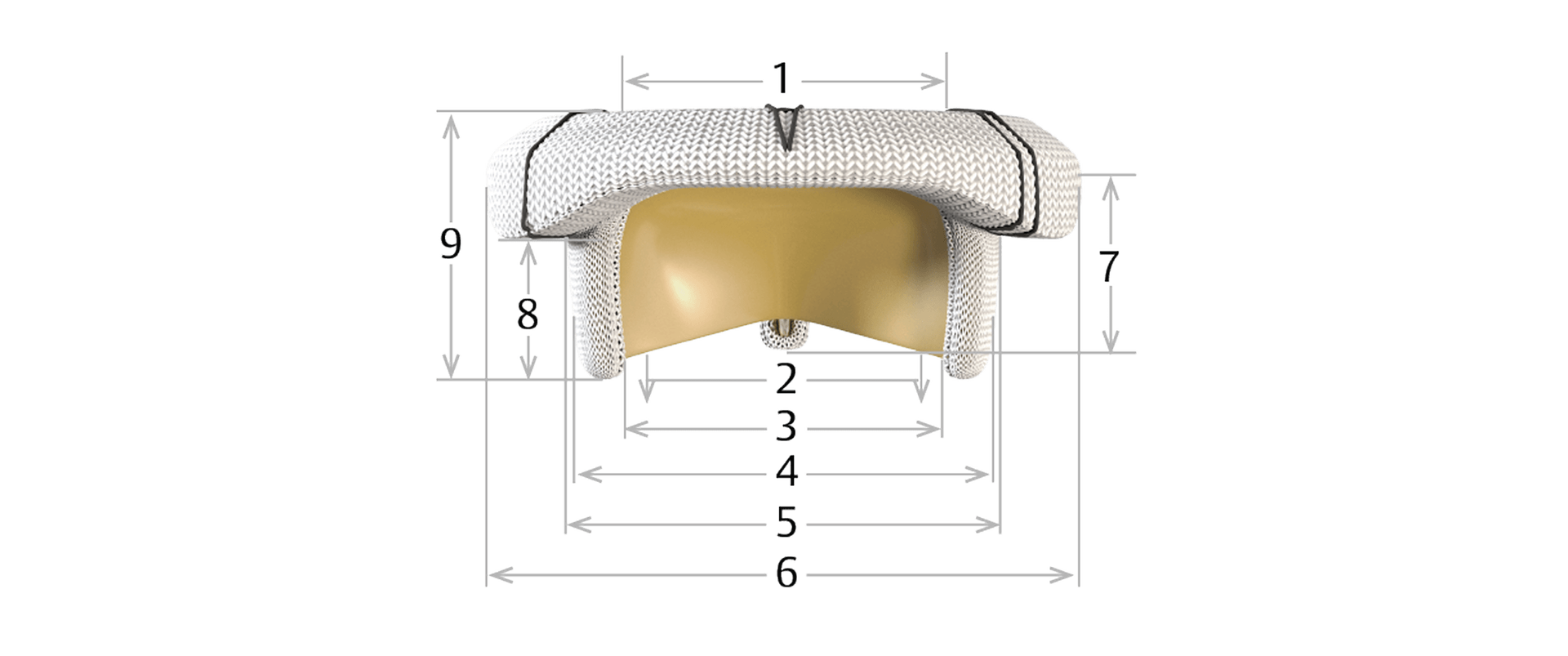
Model 11400M specifications
Sizing
| Valve Size | 25 mm | 27 mm | 29 mm | 31 mm | 33 mm |
| 1. Inflow orifice diameter (mm) | 23.0 | 25.0 | 27.0 | 29.0 | 29.0 |
| 2. Effective orifice diameter (mm)* | 19.5 | 21.0 | 23.0 | 25.0 | 25.0 |
| 3. Stent diameter (wireform, mm) | 25 | 27 | 29 | 31 | 31 |
| 4. External stent post diameter (tip, mm) | 27 | 29 | 30 | 33 | 33 |
| 5. Valve housing external diameter (mm) | 27.5 | 29.5 | 31.5 | 33.5 | 33.5 |
| 6. External sewing ring diameter (mm) | 36.5 | 38.5 | 41.0 | 42.5 | 44.5 |
| 7. Effective profile posterior (mm) | 10 | 10.5 | 11 | 11.5 | 11.5 |
| 8. Effective profile anterior (mm) | 7 | 7.5 | 8 | 8.5 | 8.5 |
| 9. Total profile height (mm) | 15 | 16 | 17 | 18 | 18 |
*Effective orifice diameter (ID-effective): a diameter derived from hydrodynamic performance data measured with a standard validated procedure. ID-effective is an indicator of hemodynamic performance.
MITRIS RESILIA Mitral Valve Brochure

Experience the RESILIA tissue portfolio difference

Important safety information
MITRIS RESILIA Mitral Valve
Indications: For use in replacement of native or prosthetic mitral heart valves.
Contraindications: There are no known contraindications with the use of the MITRIS RESILIA mitral valve.
Complications and Side Effects: Thromboembolism, valve thrombosis, hemorrhage, hemolysis, regurgitation, endocarditis, structural valve deterioration, nonstructural dysfunction, stenosis, arrhythmia, transient ischemic attack/stroke, congestive heart failure, myocardial infarction, ventricular perforation by stent posts, any of which could lead to reoperation, explantation, permanent disability, and death.
CAUTION: US law restricts this device to sale by or on the order of a physician. See Instructions for Use for full prescribing information, including indications, contraindications, warnings, precautions and adverse events.