Product support
Submit order inquiries, tech support requests

Edwards Lifesciences is with you all the way. We’re committed to the patient, cardiac surgery, and to you—the surgeon. We are adapting to the changing needs in the industry and in the world. We innovate alongside you, listening and learning – dedicated to advances that help you fight the toughest challenges in surgery.
A promise of freedom for patients and surgeons. That’s the power of RESILIA tissue. For patients, it means a life with the potential to avoid future open-heart surgery.* RESILIA tissue gives surgeons the freedom to offer a resilient tissue alternative that brings the quality-of-life benefits of tissue valves to patients.
Explore the Edwards RESILIA tissue aortic and mitral products below.

Right for today.
Ready for tomorrow.
Resilient tissue.
Ready to go.†
†Consult instructions for use for device preparation instructions
Designed to handle the pressure of the mitral position.
*No clinical data are available that evaluate the long-term impact of RESILIA tissue in patients.
Tissue with a difference
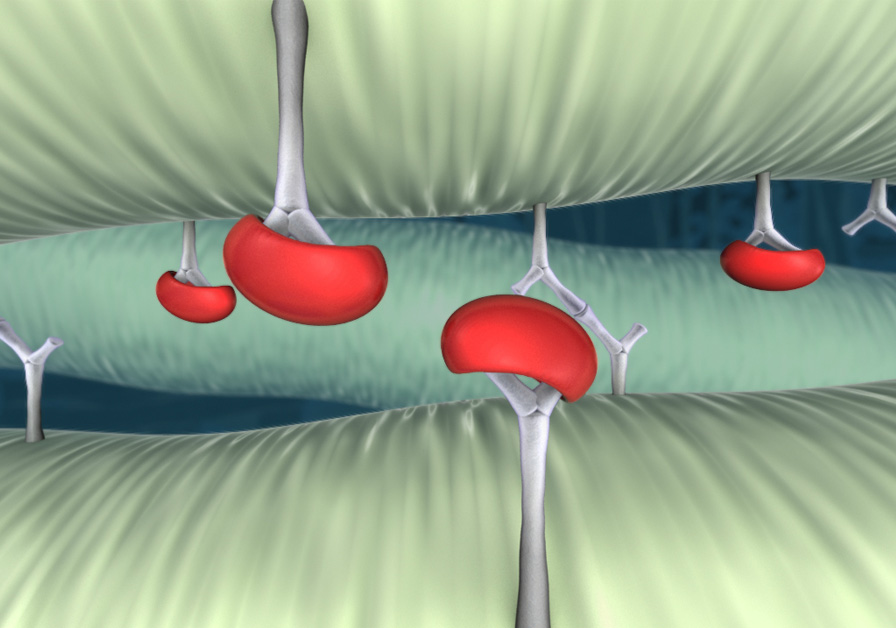
A key challenge with pericardial tissue is structural valve deterioration (SVD), much of which is due to calcium buildup. RESILIA tissue is transformed to resist calcification differently. It offers enhanced anti-calcification technology that will potentially allow the valve to last longer than conventional bioprosthetic valves.*
Visit the RESILIA tissue pre-clinical and clinical data Inspiring Results page to learn about how the tissue is performing.
*RESILIA tissue tested against tissue from commercially-available bovine pericardial valves from Edwards in a juvenile sheet model. Flameng, et al. J Thorac Cardiovasc Surg 2015;149;340-5.
**No clinical data are available that evaluate the long-term impact of RESILIA tissue in patients.

Indications: INSPIRIS RESILIA Aortic Valve - For use in replacement of native or prosthetic aortic heart valves. KONECT RESILIA Aortic Valved Conduit - For use in replacement of native or prosthetic aortic heart valves and the associated repair or replacement of a damaged or diseased ascending aorta. MITRIS RESILIA Mitral Valve - For use in replacement of native or prosthetic mitral heart valves.
Contraindications: There are no known contraindications with the use of these RESILIA tissue heart valve devices.
Complications and Side Effects: INSPIRIS RESILIA Aortic Valve - Thromboembolism, valve thrombosis, hemorrhage, hemolysis, regurgitation, endocarditis, structural valve deterioration, nonstructural dysfunction, stenosis, arrhythmia, transient ischemic attack/stroke, congestive heart failure, myocardial infarction, any of which could lead to reoperation, explantation, permanent disability, and death. Additional adverse events potentially associated with the use of polyester vascular grafts in the KONECT RESILIA AVC include hemorrhage, thrombosis, graft infection, embolism, aneurysm, pseudoaneurysm, seroma, occlusion (anastomotic intimal hyperplasia), immunological reaction to collagen (shown to be a weak immunogen; infrequent, mild, localized and self-limiting), intimal peel formation, and conduit dilatation. MITRIS RESILIA Mitral Valve - Thromboembolism, valve thrombosis, hemorrhage, hemolysis, regurgitation, endocarditis, structural valve deterioration, nonstructural dysfunction, stenosis, arrhythmia, transient ischemic attack/stroke, congestive heart failure, myocardial infarction, ventricular perforation by stent posts, any of which could lead to reoperation, explantation, permanent disability, and death.
Warnings: INSPIRIS RESILIA Aortic Valve - DO NOT ADJUST THE VALVE DIAMETER BY EXPANDING THE BAND PRIOR TO OR DURING IMPLANTATION OF THE SURGICAL VALVE. The expandable band is not designed to allow for compression or expansion during implantation of the surgical valve. This will cause damage to the valve and may result in aortic incompetence. DO NOT PERFORM STAND-ALONE BALLOON AORTIC VALVULOPLASTY PROCEDURES ON THIS VALVE FOR THE SIZES 19 – 25 mm as this may expand the valve causing aortic incompetence, coronary embolism or annular rupture. Valve-in-valve sizing in the INSPIRIS valve has only been tested with specific Edwards transcatheter heart valves. Use of other transcatheter valves may result in embolization of transcatheter devices anchored within or result in annular rupture.
Carpentier-Edwards PERIMOUNT Aortic/Mitral Bioprostheses
Indications: For use in patients whose aortic or mitral valvular disease warrants replacement of their natural or previously placed prosthetic valve.
Contraindications: Do not use if surgeon believes such would be contrary to the patient’s best interests.
Complications and Side Effects: Stenosis, regurgitation, endocarditis, hemolysis, thromboembolism, valve thrombosis, nonstructural dysfunction, structural valve deterioration, anemia, arrhythmia, hemorrhage, transient ischemic attack/ stroke, congestive heart failure, myocardial infarction, angina, ventricular perforation by stent posts (mitral valve only), any of which could lead to reoperation, explantation, permanent disability, and death.
CAUTION: Federal (USA) law restricts these devices to sale by or on the order of a physician. See instructions for use for full prescribing information.
We are committed to providing your institution, clinicians and staff with the highest levels of customer service and support to ensure seamless product implementation and ongoing use, including:
24/7 Technical support
For product information and orders