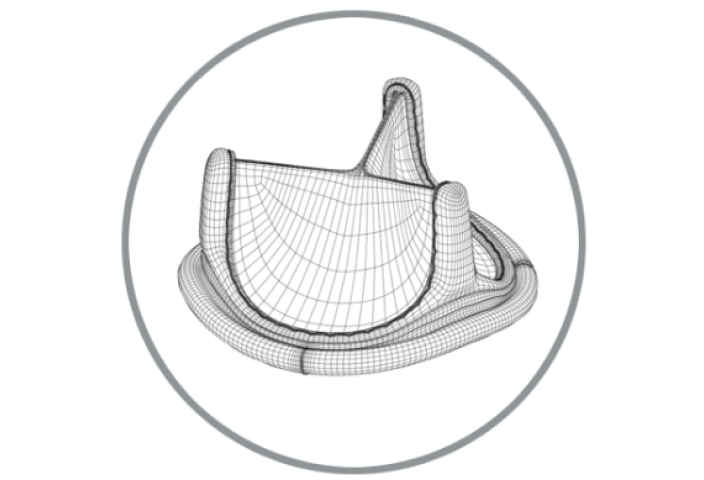
RESILIA tissue surgical valve portfolio
Discover the RESILIA tissue portfolio difference

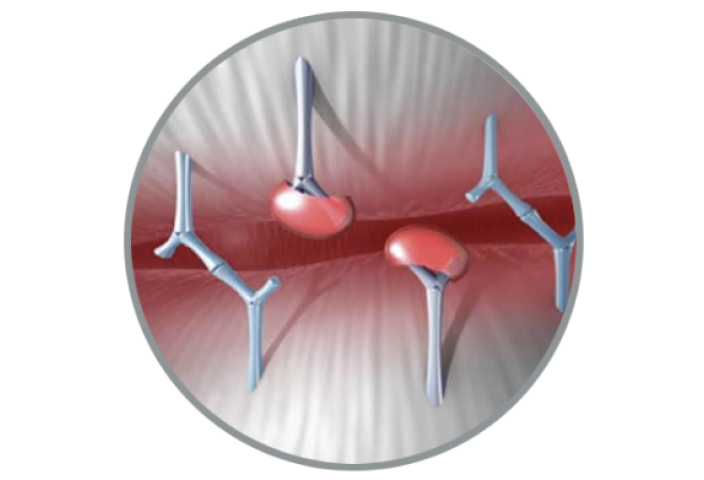
RESILIA Tissue Durability
Future Focused
Innovation built on a proven platform
RESILIA tissue valves are built on the Carpentier-Edwards PERIMOUNT valve platform, whose performance is backed by 30 years of durability data, including the largest long-term study of a bioprosthetic valve.


Innovation that transformed the tissue valve landscape
RESILIA tissue technology
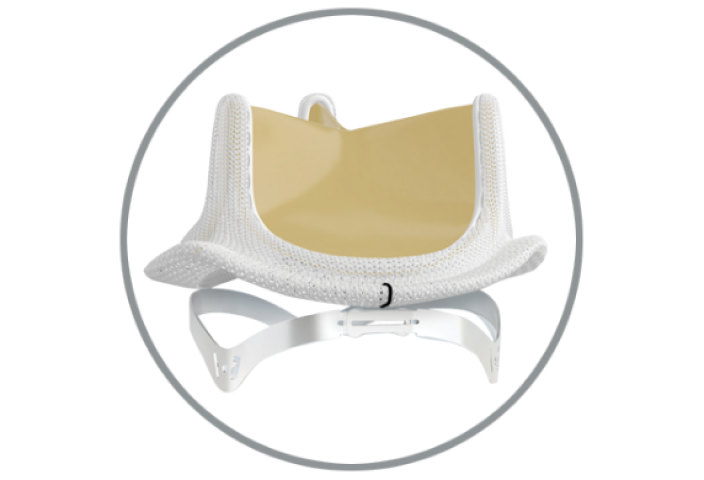
RESILIA tissue* builds on the trusted ThermaFix process and is treated with a novel preservation technology to resist calcification more effectively and allow for dry storage.1
+ Calcium-blocking technology
Stable-capping permanently blocks free aldehydes to prevent calcium binding within the tissue
+ Glycerolization
Mitigates calcium-attracting glutaraldehyde residuals and enables dry tissue storage for increased ease of use
72% less calcium content after 8 months
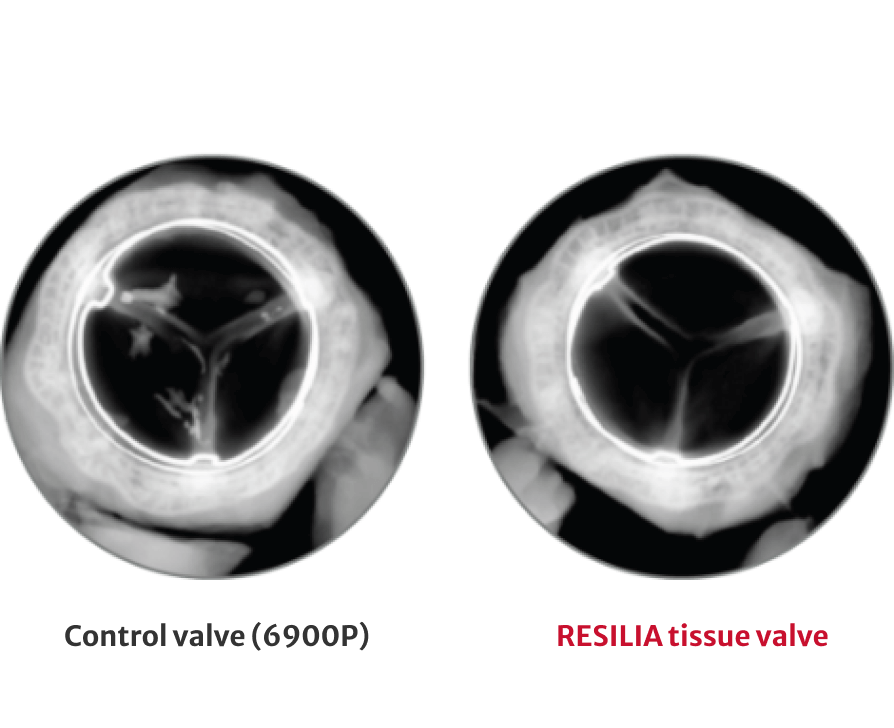
*No clinical data are available that evaluate the long-term impact of RESILIA tissue in patients.
†RESILIA tissue tested against tissue from commercially available bovine pericardial valves from Edwards in a juvenile sheep model. Flameng, et al. J Thorac Cardiovasc Surg. 2015;149:340-345.

Taking performance and durability to new heights
The RESILIA tissue surgical valve portfolio is backed by a strong, growing base of clinical evidence attesting to its hemodynamic performance and durability. RESILIA tissue treatment has been applied to INSPIRIS and MITRIS valves and the KONECT aortic valved conduit.


Clinically stable hemodynamics2,3


Durability data2,3


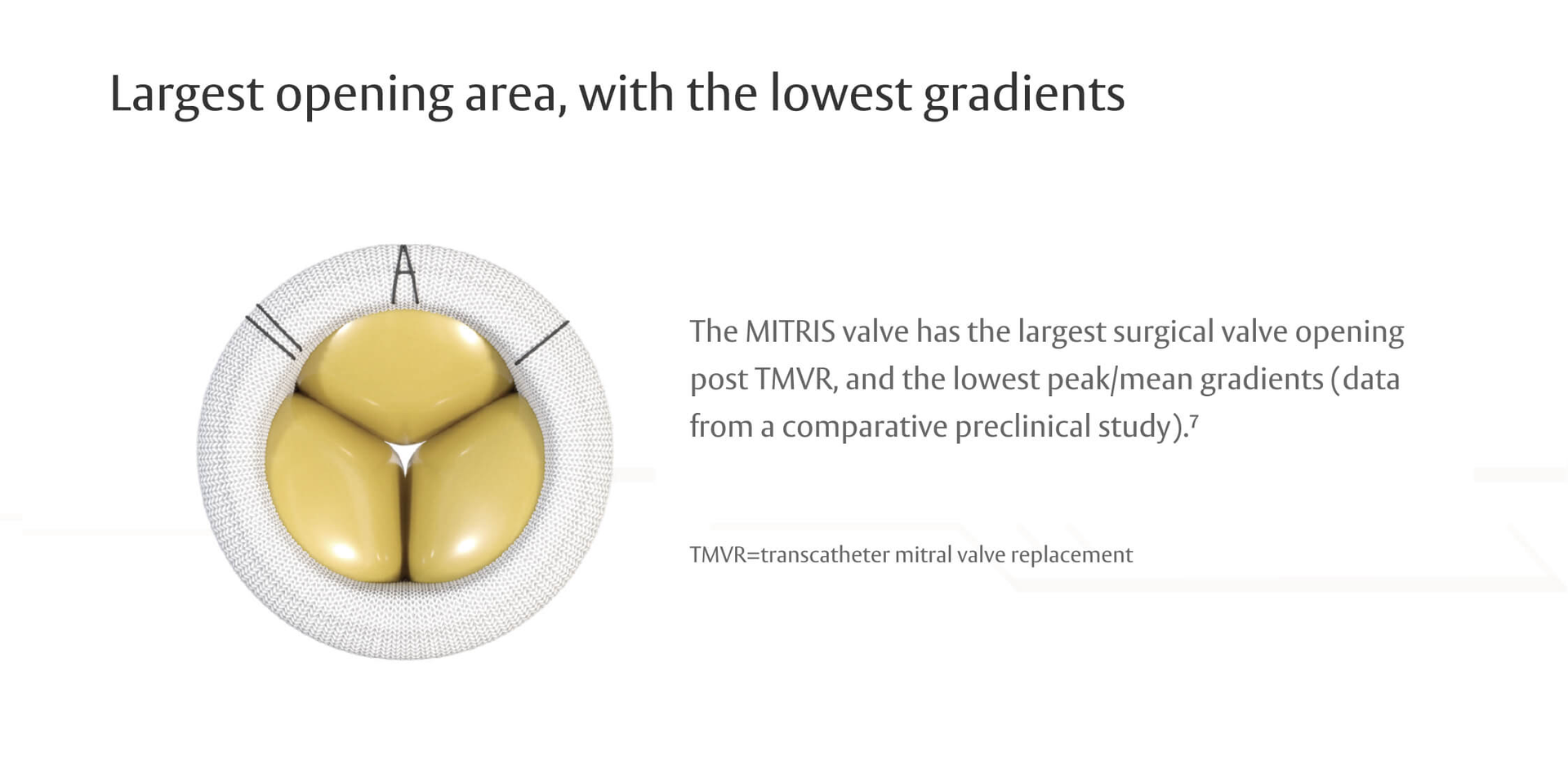
RESILIA tissue valves showed less SVD-related HVD vs conventional valves in a comparison of COMMENCE and PARTNER IIA trials4
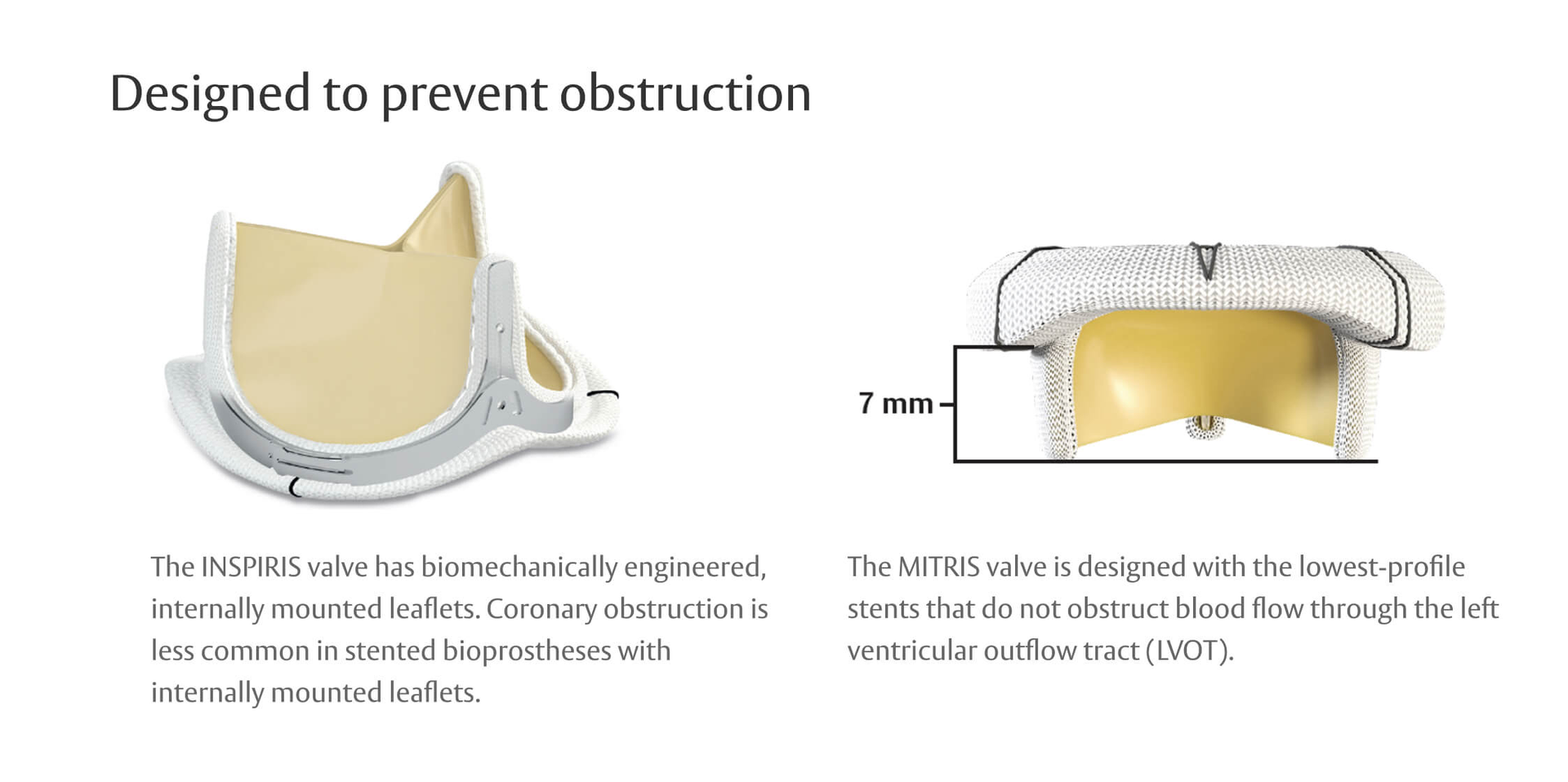
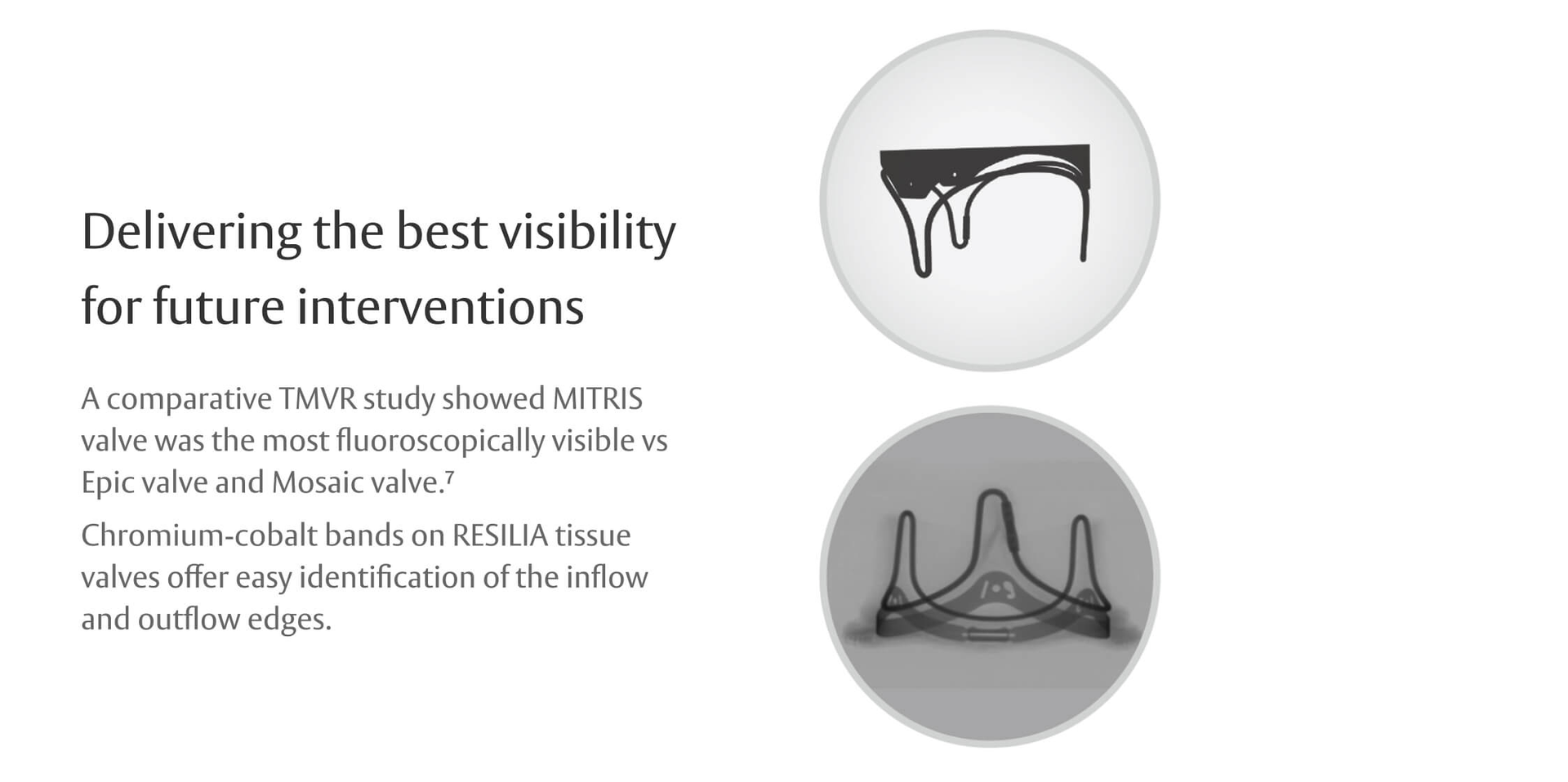
Innovation that keeps future procedures in mind
Featured products
Explore our current RESILIA tissue surgical valve portfolio
References
- Flameng W, Hermans H, Verbeken E, et al. A randomized assessment of an advanced tissue preservation technology in the juvenile sheep model. J Thorac Cardiovasc Surg. 2015;149(1):340-345.
- Beaver T, Bavaria J, Griffith B, et al. Seven-year outcomes following aortic valve replacement with a novel tissue bioprosthesis. J Thorac Cardiovasc Surg. 2023;x:1-11.
- Heimansohn DA, Baker C, Rodriguez E, et al. Mid-term outcomes of the COMMENCE trial investigating mitral valve replacement using a bioprosthesis with a novel tissue. JTCVS Open. 2023;15:151-163.
- Bartus K, Bavaria J, Thourani V, et al. Structural hemodynamic valve deterioration durability of RESILIA-tissue versus contemporary aortic bioprostheses. J Comp Eff Res. 2023;12(3):e220180.
- Saxon JT, Allen K, Cohen D, et al. Bioprosthetic valve fracture during valve-in-valve TAVR: bench to bedside. Interv Cardiol. 2018;13(1):20-26.
- Saxon JT, Allen K, Cohen D, et al. Complications of bioprosthetic valve fracture as an adjunct to valve-in-valve TAVR. Structural Heart. 2019;3(2):92-99.
- Wang DD, O’Neill B, Caranasos T, et al. Comparative differences of mitral valve-in-valve implantation: A new mitral bioprosthesis versus current mosaic and epic valves. Catheter Cardiovasc Interv. 2022;99(3):934-942.
Important Safety Information
RESILIA Tissue Devices
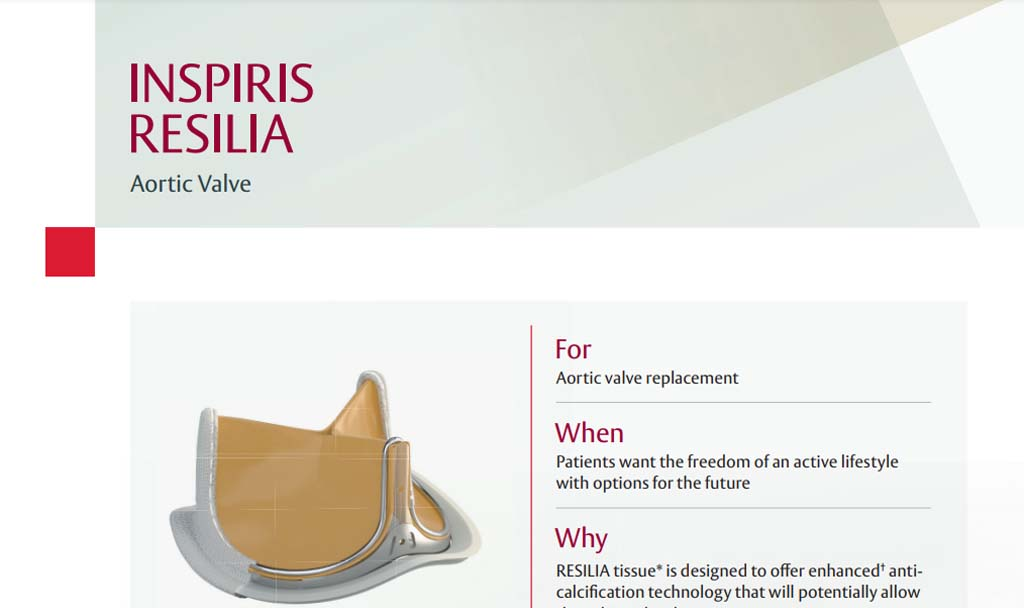
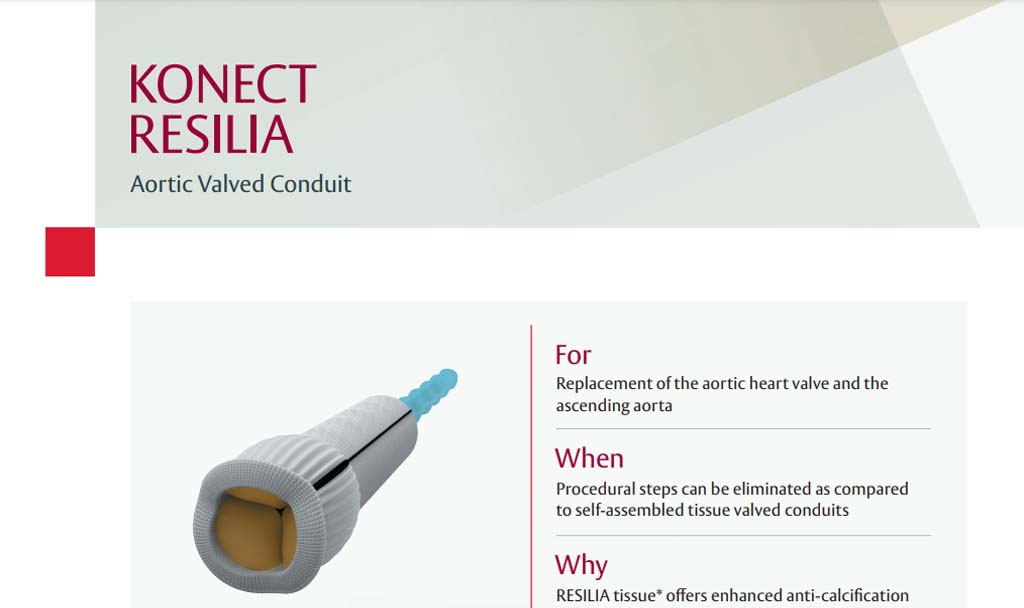
Indications: INSPIRIS RESILIA Aortic Valve - For use in replacement of native or prosthetic aortic heart valves. KONECT RESILIA Aortic Valved Conduit - For use in replacement of native or prosthetic aortic heart valves and the associated repair or replacement of a damaged or diseased ascending aorta. MITRIS RESILIA Mitral Valve - For use in replacement of native or prosthetic mitral heart valves.
Contraindications: There are no known contraindications with the use of these RESILIA tissue heart valve devices.
Complications and Side Effects: INSPIRIS RESILIA Aortic Valve - Thromboembolism, valve thrombosis, hemorrhage, hemolysis, regurgitation, endocarditis, structural valve deterioration, nonstructural dysfunction, stenosis, arrhythmia, transient ischemic attack/stroke, congestive heart failure, myocardial infarction, any of which could lead to reoperation, explantation, permanent disability, and death. Additional adverse events potentially associated with the use of polyester vascular grafts in the KONECT RESILIA AVC include hemorrhage, thrombosis, graft infection, embolism, aneurysm, pseudoaneurysm, seroma, occlusion (anastomotic intimal hyperplasia), immunological reaction to collagen (shown to be a weak immunogen; infrequent, mild, localized and self-limiting), intimal peel formation, and conduit dilatation. MITRIS RESILIA Mitral Valve - Thromboembolism, valve thrombosis, hemorrhage, hemolysis, regurgitation, endocarditis, structural valve deterioration, nonstructural dysfunction, stenosis, arrhythmia, transient ischemic attack/stroke, congestive heart failure, myocardial infarction, ventricular perforation by stent posts, any of which could lead to reoperation, explantation, permanent disability, and death.
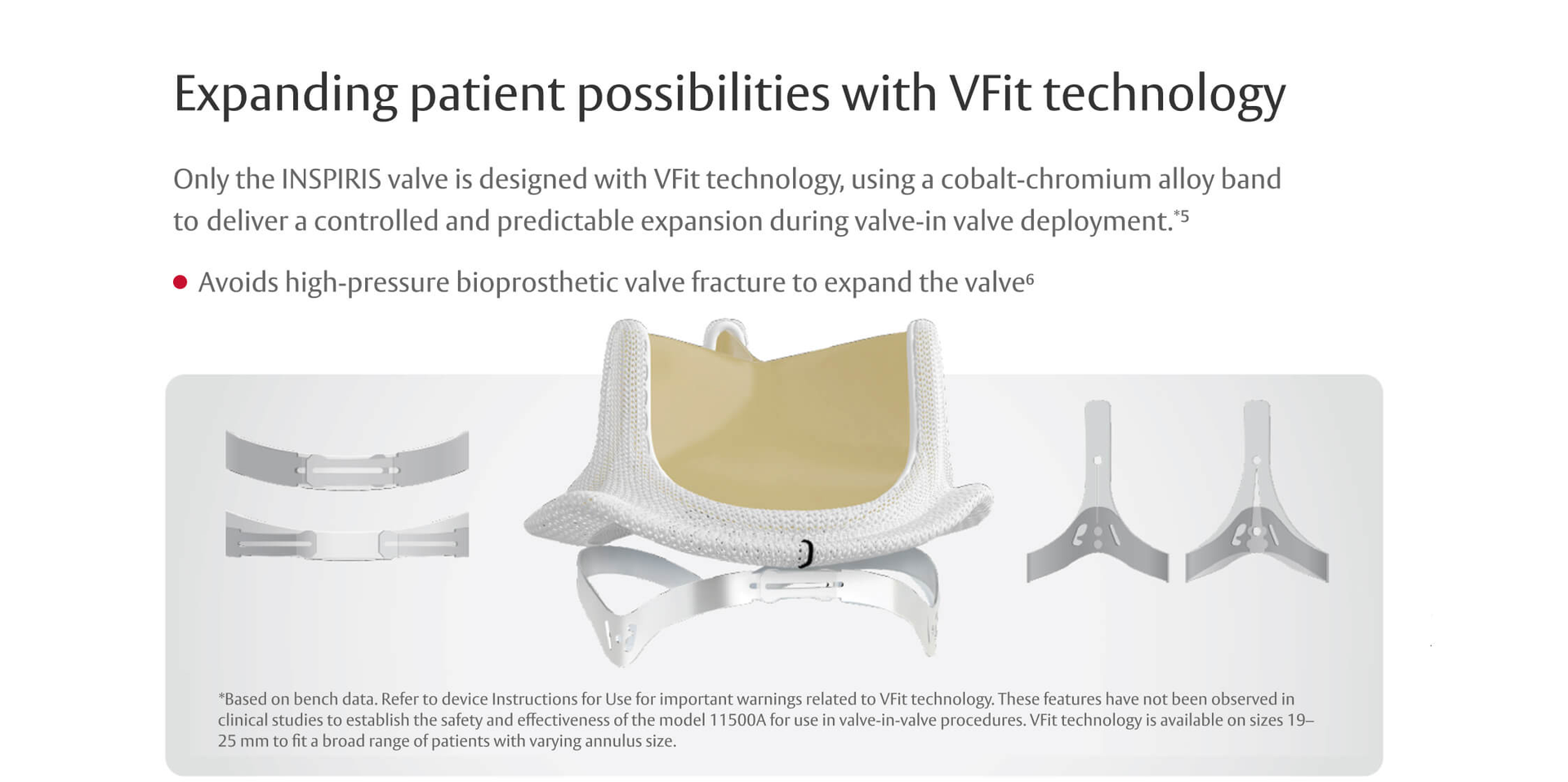
Warnings: INSPIRIS RESILIA Aortic Valve - DO NOT ADJUST THE VALVE DIAMETER BY EXPANDING THE BAND PRIOR TO OR DURING IMPLANTATION OF THE SURGICAL VALVE. The expandable band is not designed to allow for compression or expansion during implantation of the surgical valve. This will cause damage to the valve and may result in aortic incompetence. DO NOT PERFORM STAND-ALONE BALLOON AORTIC VALVULOPLASTY PROCEDURES ON THIS VALVE FOR THE SIZES 19 – 25 mm as this may expand the valve causing aortic incompetence, coronary embolism or annular rupture. Valve-in-valve sizing in the INSPIRIS valve has only been tested with specific Edwards transcatheter heart valves. Use of other transcatheter valves may result in embolization of transcatheter devices anchored within or result in annular rupture.
CAUTION: Federal (USA) law restricts these devices to sale by or on the order of a physician. See instructions for use for full prescribing information.