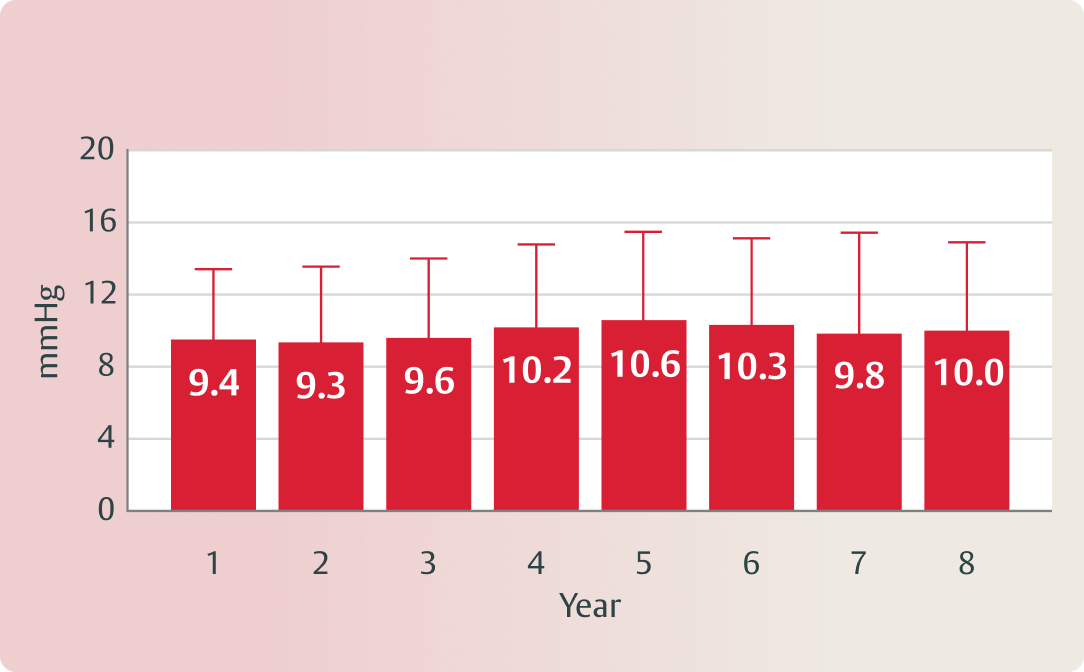
8-year SAVR outcomes of RESILIA tissue valves 1
(Echo-derived mean gradient, mmHg)
Clinical evidence supports RESILIA tissue performance
Since 2004, RESILIA tissue has been extensively studied. The growing body of clinical evidence supports RESILIA tissue valves' hemodynamic performance and resistance to structural valve deterioration in the mitral position.1,2

(Echo-derived mean gradient, mmHg)
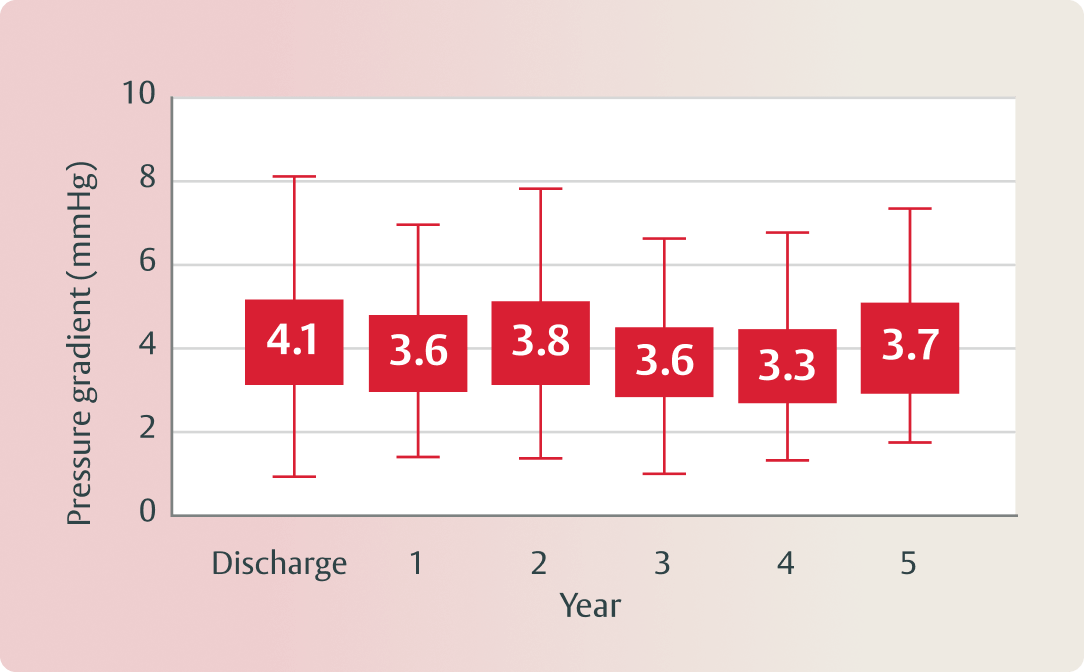
(Echo-derived median gradient, mmHg)
Clinically stable hemodynamics and one incident of structural valve deterioration (SVD) through 5 years.
Reduced calcification after 8 months, exceeding the 5-month reporting requirement
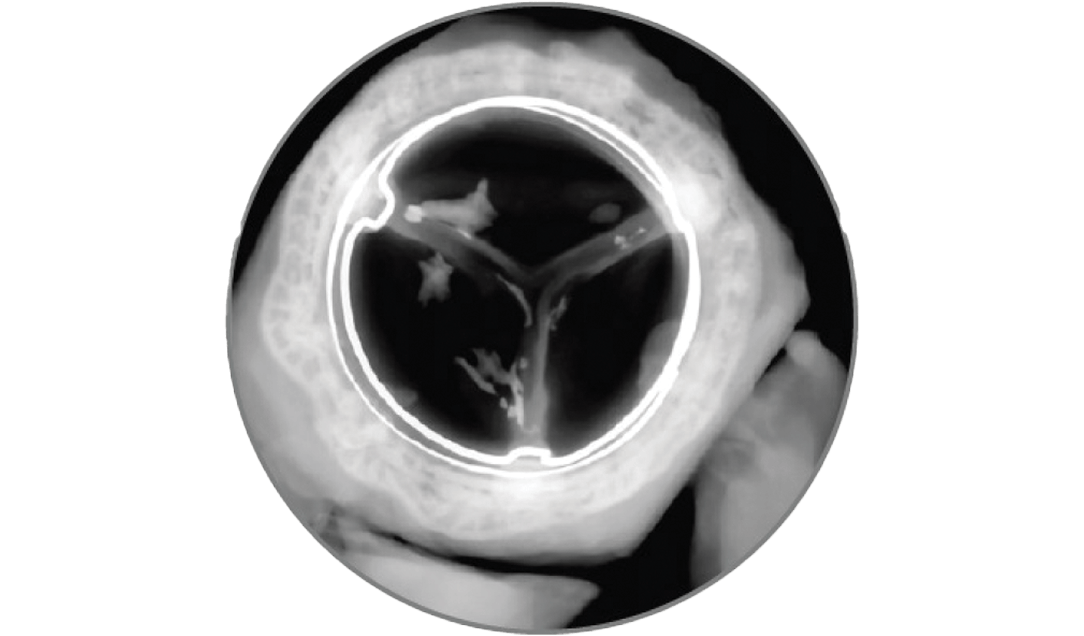
This global study is planned to enroll up to 500 patients implanted with the MITRIS RESILIA valve

Learn more about the promising results and conclusions of the various RESILIA tissue studies
See the valve dimensions, sizing, accessories, and resources.
Indications: For use in replacement of native or prosthetic mitral heart valves.
Contraindications: There are no known contraindications with the use of the MITRIS RESILIA mitral valve.
Complications and Side Effects: Thromboembolism, valve thrombosis, hemorrhage, hemolysis, regurgitation, endocarditis, structural valve deterioration, nonstructural dysfunction, stenosis, arrhythmia, transient ischemic attack/stroke, congestive heart failure, myocardial infarction, ventricular perforation by stent posts, any of which could lead to reoperation, explantation, permanent disability, and death.
CAUTION: US law restricts this device to sale by or on the order of a physician. See Instructions for Use for full prescribing information, including indications, contraindications, warnings, precautions and adverse events.