MITRIS RESILIA Mitral Valve

Designed for the future
The MITRIS RESILIA valve was made with future transcatheter valve-in-valve (ViV) interventions in mind*1
Better visibility under fluoroscopy
Compared to commercially available valves in a pre-clinical study, the MITRIS valve has:

- a columnar shape design that enables more uniformity in transcatheter heart valve (THV) expansion*1
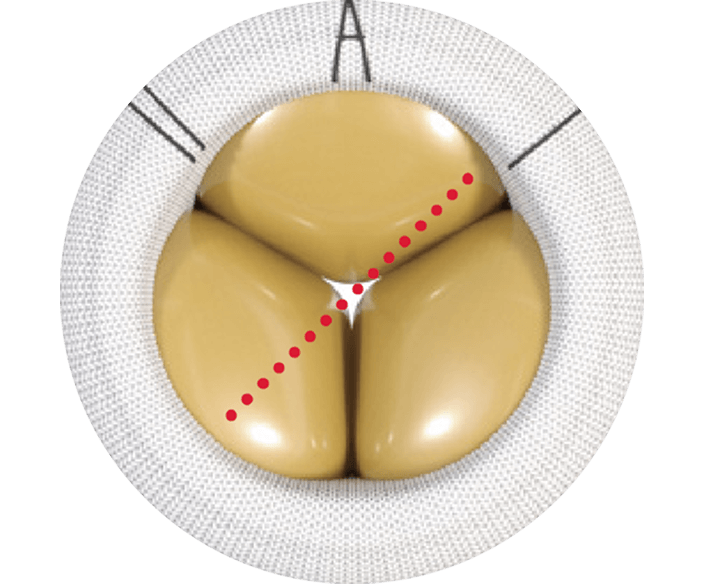
- the least deformation of the THV frame for Transcatheter Mitral Valve Replacement (TMVR) valve-in-valve (ViV)*1
- the greatest THV leaflet opening area and lowest peak/mean trans-mitral gradient for TMVR ViV*1
*The safety and effectiveness of ViV in the MITRIS valve has not been established.
Clinical data
See how clinical evidence supports RESILIA tissue valves’ hemodynamic performance

References
- Wang DD, O’Neill BP, Caranasos TG, et al. Comparative differences of mitral valve-in-valve implantation: A new mitral bioprosthesis versus current mosaic and epic valves. Catheter Cardiovasc Interv. 2022;99(3):934-942. doi: 10.1002/ccd.30011
Important safety information
MITRIS RESILIA Mitral Valve
Indications: For use in replacement of native or prosthetic mitral heart valves.
Contraindications: There are no known contraindications with the use of the MITRIS RESILIA mitral valve.
Complications and Side Effects: Thromboembolism, valve thrombosis, hemorrhage, hemolysis, regurgitation, endocarditis, structural valve deterioration, nonstructural dysfunction, stenosis, arrhythmia, transient ischemic attack/stroke, congestive heart failure, myocardial infarction, ventricular perforation by stent posts, any of which could lead to reoperation, explantation, permanent disability, and death.
CAUTION: US law restricts this device to sale by or on the order of a physician. See Instructions for Use for full prescribing information, including indications, contraindications, warnings, precautions and adverse events.