- Designed for vascular vessel repair
- XenoLogiX* two-step processing treatment for performance
- Ready to use after standard rinse duration
- Easy to suture with suture retention
Duravess bovine pericardial vascular patch


Duravess bovine pericardial vascular patch
A bovine pericardial vascular patch, born from 30+ years of bovine tissue expertise.
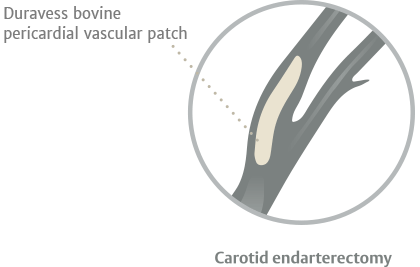
Edwards Lifesciences, the long-established bovine tissue technology leader, manufactures the Duravess bovine pericardial vascular patch designed for vessel repair. With more than 30 years of bovine tissue expertise and the proven performance of tissue processed through Edwards proprietary XenoLogiX* technology for surgical heart valves, the Duravess bovine pericardial vascular patch is a viable solution for vascular procedures.
With a history of celebrated products that includes the revolutionary Fogarty catheter, Edwards continues to work with a commitment to provide vascular surgeons with reliable solutions, from a company they can trust.
Performance
The Duravess bovine pericardial vascular patch is intended for use as a surgical patch material for vascular reconstruction and repairs, peripheral vascular reconstruction and repairs, and suture-line buttressing.
Flexible and easy to handle
- Tissue thickness: 0.254 mm to 0.762 mm
- Bovine pericardial tissue contains a high amount of structural protein with elastic properties that allow conformity to challenging anatomy
- Bovine pericardial patches are reported to show a significant decrease in intraoperative suture line bleeding compared to Dacron
The Duravess bovine pericardial vascular patch can be used in the following common procedures:
- Carotid endarterectomy
- Profundaplasty
- Arteriovenous access revisions
- Femoral, iliac, renal and tibial endarterectomy
* No clinical data are available that evaluate the long-term impact of the XenoLogiX treatment in patients.
Models
Available in three sizes specific to vascular use, and easily trimmed to fit.
| Model | Dimension | Image |
| DP1X6 | 1 cm x 6 cm |  |
| DP08X8 | 0.8 cm x 8 cm |  |
| DP2X9 | 2 cm x 9 cm |  |
Important safety information
CAUTION: Federal (United States) law restricts this device to sale by or on the order of a physician.
See Instructions For Use (IFU) / Directions For Use (DFU) for full prescribing information, including indications, contraindications, warnings, precautions and adverse events.


