PASCAL implant
Designed to reduce leaflet stress and increase open orifice area enabling low gradients
For degenerative mitral regurgitation
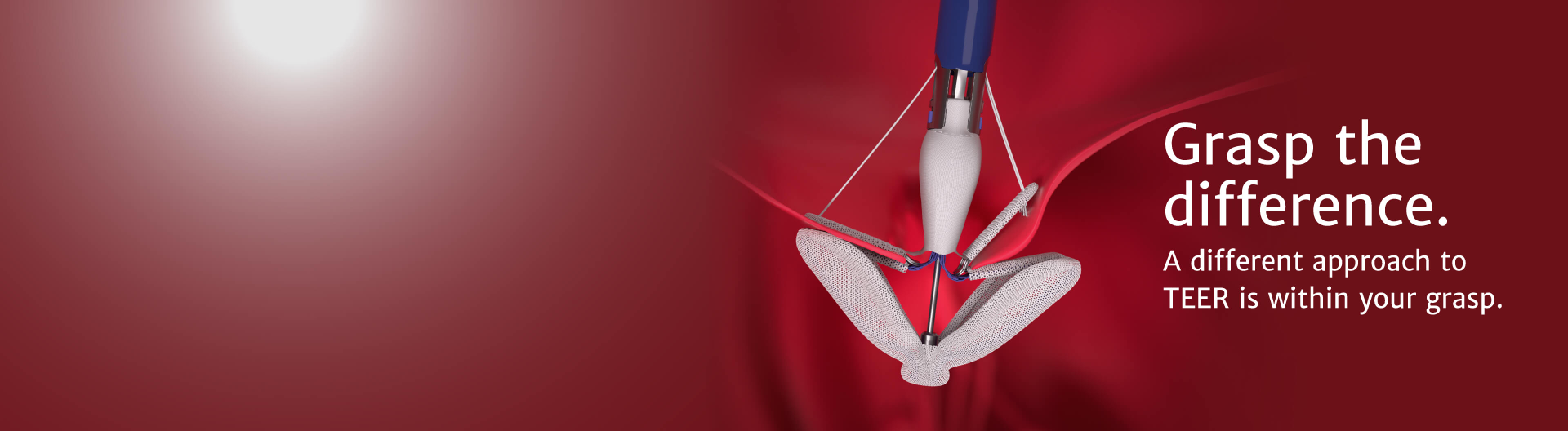
The latest advancement from Edwards Lifesciences in transcatheter mitral therapies: The PASCAL Precision system
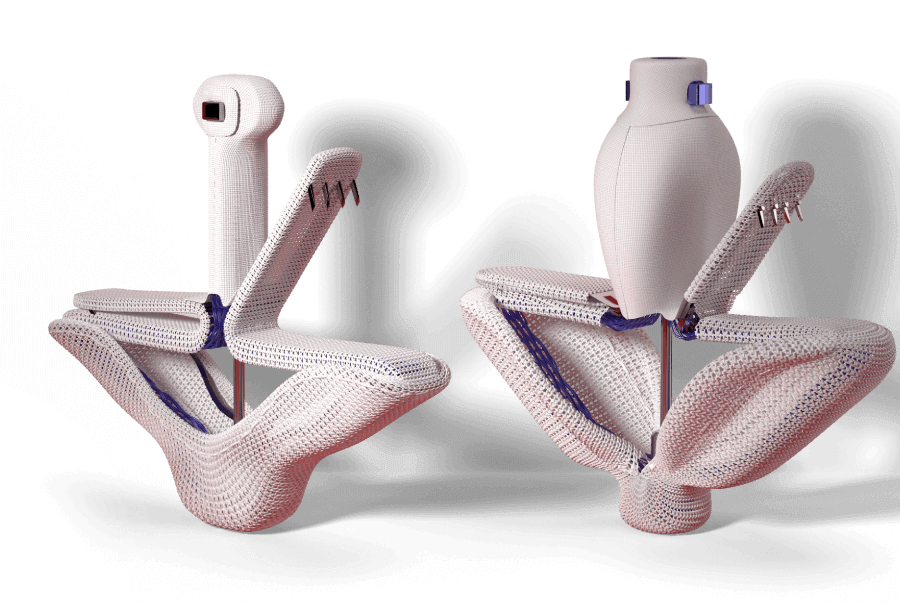
Designed to reduce leaflet stress and increase open orifice area enabling low gradients
Features a narrower design profile to help you optimize your treatment of degenerative mitral regurgitation

Treat your patients with degenerative mitral regurgitation with the latest transcatheter mitral innovation from Edwards Lifesciences
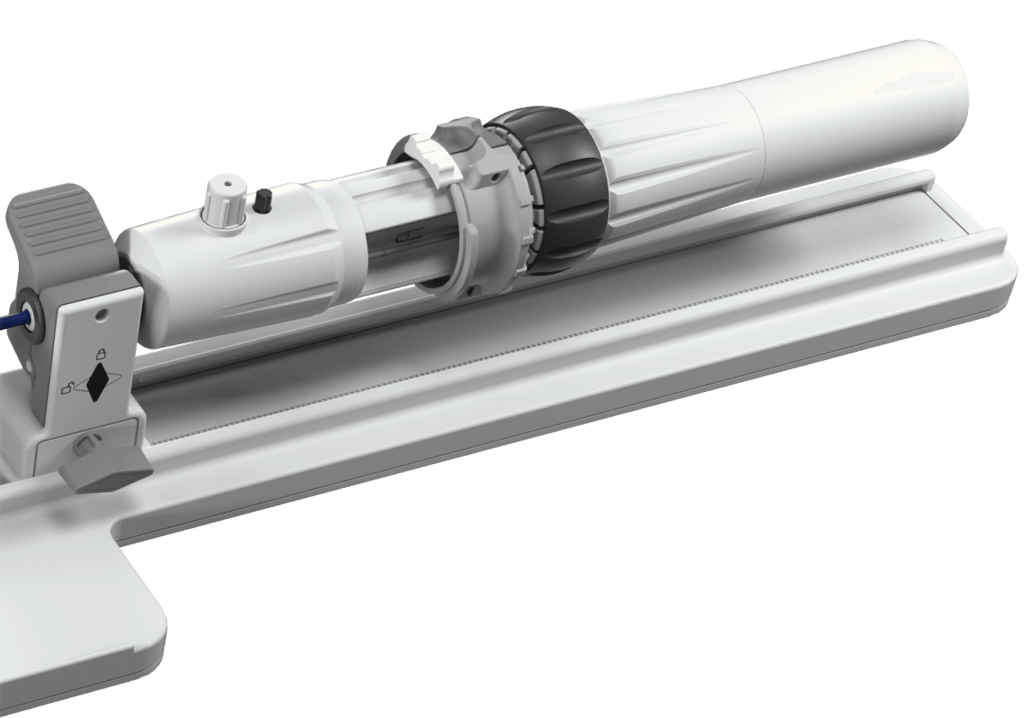
Deploy the implant with confidence



N=141 patients (n=59 DMR patients) were included in a retrospective multi-center observational study1 conducted at three sites in Germany to assess change in residual MR pre- and post-delivery of the PASCAL device (PASCAL and PASCAL Ace implants). Change in pre- and post-delivery MR was evaluated from intraprocedural echo in a blinded assessment at a central core lab, and classified into either improved, unchanged, or deteriorated.+
Of the DMR subset of the study cohort* (n=59 patients):

1 Mahabadi, A. (2024, May 14-17). Predictable implant release: evidence, and case-based discussion [Conference presentation]. Euro PCR 2024, Paris, France.
+ Deteriorated defined as: reoccurrence of MR due to new jet, increase of residual MR, and/or return of MR
* The PASCAL Precision System is approved for DMR only in the US

CLASP IID Randomized Control Trial (RCT): The first and only RCT to directly compare two contemporary TEER therapy in patients with DMR.
CLASP IID Registry: A first-of-its-kind registry to evaluate the PASCAL system in DMR patients with complex anatomy.
CLASP: The first international, multicenter study to evaluate the safety and clinical outcomes of the PASCAL system.

The objective of this prospective, multicenter, randomized, controlled pivotal trial is to evaluate safety and effectiveness in patients with degenerative mitral regurgitation (DMR) who have been determined to be at prohibitive risk for mitral valve surgery by the Heart Team.
of patients sustained MR ≤ 1+ at 2 years with the PASCAL system1

freedom from cardiovascular mortality at 2 years with the PASCAL system

freedom from HFH at 2 years with the PASCAL system

of patients achieved NYHA Class I/II at 2 years with the PASCAL system
Graph shows unpaired analysis for NYHA functional class (mean ±SD). P values for intragroup comparison for NYHA were calculated from paired analysis using the Wilcoxon signed rank test. P values for intergroup comparison of NYHA functional class I/II were calculated using Fisher exact test.

point improvement from baseline to 2 years with the PASCAL system
Graph shows unpaired analysis for KCCQ (mean ±SD). P value for intergroup comparison of KCCQ score was calculated using the analysis of covariance (ANCOVA) model adjusted for baseline values and planned treatment as covariates.


MR Reduction

Freedom from cardiovascular mortality

Freedom from heart failure hospitalization

New York Heart Association Functional Class

KCCQ Score
of patients sustained MR ≤ 1+ at 2 years with the PASCAL system1
freedom from cardiovascular mortality at 2 years with the PASCAL system
freedom from HFH at 2 years with the PASCAL system
of patients achieved NYHA Class I/II at 2 years with the PASCAL system
Graph shows unpaired analysis for NYHA functional class (mean ±SD). P values for intragroup comparison for NYHA were calculated from paired analysis using the Wilcoxon signed rank test. P values for intergroup comparison of NYHA functional class I/II were calculated using Fisher exact test.
point improvement from baseline to 2 years with the PASCAL system
Graph shows unpaired analysis for KCCQ (mean ±SD). P value for intergroup comparison of KCCQ score was calculated using the analysis of covariance (ANCOVA) model adjusted for baseline values and planned treatment as covariates.
1. Zahr F, et al. CLASP IID Randomized Trial and Registry: Two-Year Outcomes of Transcatheter Edge-to-Edge Repair for Degenerative Mitral Regurgitation. Presented at: TCT Annual Congress; 2024 Oct 30; Washington, DC.

CLASP IID is a clinical trial from Edwards Lifesciences, reinforcing TEER as safe and effective for the treatment of patients with degenerative mitral regurgitation.
The objective of this prospective, multinational registry within the construct of the CLASP IID trial is to study significant symptomatic DMR patients with complex mitral valve anatomy deemed suitable for treatment with the PASCAL system.
of patients sustained MR ≤2 + at 2 years with the PASCAL system

freedom from cardiovascular mortality at 2 years with the PASCAL system

KCCQ improvement from baseline to 2 years with the PASCAL system

of patients achieved NYHA Class I/II at 2 years with the PASCAL system


MR Reduction

Freedom from cardiovascular mortality

KCCQ Score

New York Heart Association Functional Class
of patients sustained MR ≤2 + at 2 years with the PASCAL system
freedom from cardiovascular mortality at 2 years with the PASCAL system
KCCQ improvement from baseline to 2 years with the PASCAL system
of patients achieved NYHA Class I/II at 2 years with the PASCAL system
1. Zahr F, et al. CLASP IID Randomized Trial and Registry: Two-Year Outcomes of Transcatheter Edge-to-Edge Repair for Degenerative Mitral Regurgitation. Presented at: TCT Annual Congress; 2024 Oct 30; Washington, DC.
The first, international multicenter study to evaluate the PASCAL system provides 3-year outcomes in patients with clinically significant MR. The results in this section are for the patients in this study with degenerative mitral regurgitation.
of DMR patients sustained MR ≤ 2+ at 3 years with the PASCAL system3

freedom from all-cause mortality at 3 years with the PASCAL system

freedom from HFH at 3 years with the PASCAL system

of DMR patients achieved NYHA Class I/II at 3 years with the PASCAL system


DMR

Survival

Freedom from HF rehospitalization

New York Heart Association Functional Class
of DMR patients sustained MR ≤ 2+ at 3 years with the PASCAL system3
freedom from all-cause mortality at 3 years with the PASCAL system
freedom from HFH at 3 years with the PASCAL system
of DMR patients achieved NYHA Class I/II at 3 years with the PASCAL system
2. Spargias K et al. Three-year outcomes for the transcatheter repair in patients with mitral regurgitation from the CLASP study. CCI. 2023.


Indications: The PASCAL Precision transcatheter valve repair system (the PASCAL Precision system) is indicated for the percutaneous reduction of significant, symptomatic mitral regurgitation (MR ≥ 3+) due to primary abnormality of the mitral apparatus (degenerative MR) in patients who have been determined to be at prohibitive risk for mitral valve surgery by a heart team, which includes a cardiac surgeon experienced in mitral valve surgery and a cardiologist experienced in mitral valve disease, and in whom existing comorbidities would not preclude the expected benefit from reduction of the MR.
Contraindications: The PASCAL Precision system is contraindicated in patients with the following conditions: patients who cannot tolerate procedural anticoagulation or post procedural anti-platelet regimen; untreatable hypersensitivity or contraindication to nitinol alloys (nickel and titanium) or contrast media; active endocarditis of the mitral valve; rheumatic etiology for mitral regurgitation; evidence of intracardiac, inferior vena cava (IVC) or femoral venous thrombus.
Warnings: The devices are designed, intended, and distributed for single use only. There are no data to support the sterility, non-pyrogenicity, and functionality of the devices after reprocessing. Devices should be handled using standard sterile technique to prevent infection. Do not expose any of the devices to any solutions, chemicals, etc., except for the sterile physiological and/or heparinized saline solution. Irreparable damage to the device, which may not be apparent under visual inspection, may result. Do not use any of the devices in the presence of combustible or flammable gases, anesthetics, or cleaners/disinfectants. Do not use the devices if the expiration date has elapsed. Do not use if the packaging seal is broken or if the packaging is damaged for sterile devices. Do not use if any of the devices were dropped, damaged or mishandled in any way. Standard flushing and de-airing technique should be used during preparation and throughout procedure to prevent air embolism.
As with any implanted medical device, there is a potential for an adverse immunological response. Serious adverse events, sometimes leading to surgical intervention and/or death, may be associated with the use of this system ("Potential Adverse Events"). A full explanation of the benefits and risks should be given to each prospective patient before use. Careful and continuous medical follow-up is advised so that implant-related complications can be diagnosed and properly managed. Anticoagulation therapy must be determined by the physician per institutional guidelines.
Precautions: Prior to use, patient selection should be performed by a heart team to assess patient risk and anatomical suitability. After use, short-term anticoagulation therapy may be necessary after valve repair with the PASCAL Precision system. Prescribe anticoagulation and other medical therapy per institutional guidelines
Potential Adverse Events: Below is a list of the potential adverse effects (e.g., complications) associated with the use of the PASCAL Precision system: death; abnormal lab values; allergic reaction to anesthetic, contrast, heparin, Nitinol; anemia or decreased hemoglobin (may require transfusion); aneurysm or pseudoaneurysm; angina or chest pain; anaphylactic shock; arrhythmias – atrial (i.e. atrial fibrillation, Supraventricular tachycardia); arrhythmias – ventricular (i.e. ventricular tachycardia, ventricular fibrillation); arterio-venous fistula; atrial septal injury requiring intervention; bleeding; cardiac arrest; cardiac failure; cardiac injury, including perforation; cardiac tamponade/pericardial effusion; cardiogenic shock; chordal entanglement or rupture that may require intervention; coagulopathy, coagulation disorder, bleeding diathesis; conduction system injury which may require permanent pacemaker; deep vein thrombosis (DVT); deterioration of native valve (e.g., leaflet tearing, retraction, thickening); dislodgement of previously deployed implant; dyspnea; edema; electrolyte imbalance; emboli/embolization including air, particulate, calcific material, or thrombus; endocarditis; esophageal irritation; esophageal perforation or stricture; exercise intolerance or weakness; failure to retrieve any PASCAL Precision system components; fever; gastrointestinal bleeding or infarct; heart failure; hematoma; hemodynamic compromise; hemolysis; hemorrhage requiring transfusion or intervention; hypertension; hypotension; implant deterioration (wear, tear, fracture, or other); implant embolization; implant malposition or failure to deliver to intended site; implant migration; implant thrombosis; infection; inflammation; LVOT obstruction; mesenteric ischemia; multi-system organ failure; myocardial infarction; native valve injury; native valve stenosis; nausea and/or vomiting; need for open surgery (conversion, emergent or nonemergent reoperation, explant), nerve injury neurological symptoms, including dyskinesia, without diagnosis of TIA or stroke; non-neurological thromboembolic events; pain; papillary muscle damage; paralysis; PASCAL Precision system component(s) embolization; peripheral ischemia; permanent disability; pleural effusion; pulmonary edema; pulmonary embolism; reaction to anti-platelet or anticoagulation agents; renal failure; renal insufficiency; respiratory compromise, respiratory failure, atelectasis, pneumonia – may require prolonged ventilation; retroperitoneal bleed; septal damage or perforation; septicemia, sepsis; skin burn, injury or tissue changes due to exposure to ionizing radiation; single leaflet device attachment (SLDA); stroke; syncope; transient ischemic attack (TIA); urinary tract infection and/or bleeding; valvular regurgitation; vascular injury or trauma, including dissection or occlusion; vessel spasm; ventricular wall damage or perforation; worsening native valve regurgitation / valvular insufficiency; worsening of heart failure; wound dehiscence, delayed or incomplete healing.
CAUTION: Federal (United States) law restricts these devices to sale by or on the order of a physician. See instructions for use for full prescribing information.
For more information, see Edwards.com/PASCAL