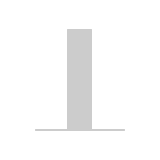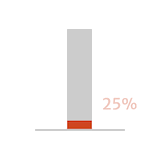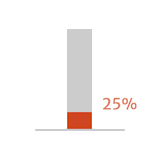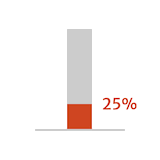
- of patients with symptomatic (NYHA
class III/IV) moderately severe or severe
aortic regurgitation (AR) could die
within 1 year of diagnosis
A heart‐to‐heart conversation could put your patients on a better path.

Aortic valve disease symptoms may not appear until the condition is severe, and waiting for symptoms before treatment could lead to more serious complications and even death.1

Adapted from Braunwald 2018.

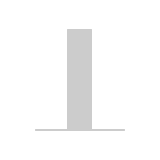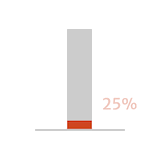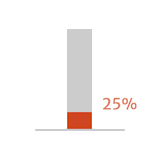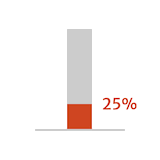
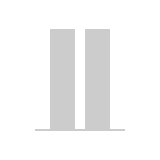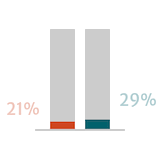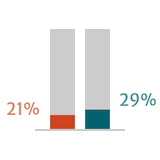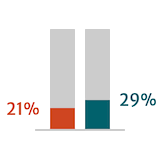

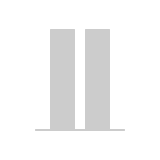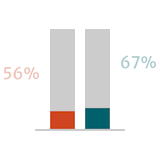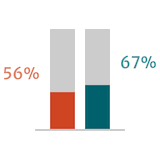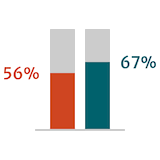
Guidelines suggest that postoperative outcomes in patients with severe AR are better when surgery is performed early or before the onset of symptoms.6
You know what matters most to your patients, but they may not know how important it is to consider those things when selecting a heart valve. With your help, patients can make more educated choices on what valve is best for them and their lifestyle.
When adequate information and tools are provided to patients to support the shared decision‐making process in valve selection, it can serve to:7
Vitamin K antagonists (VKAs), such as warfarin, are the recommended anticoagulation therapy for patients with mechanical valves. However, to be successful, VKAs require patient education and adherence to attain and maintain a therapeutic international normalized ratio (INR).
The challenges of lifetime anticoagulant therapy for patients may include:6

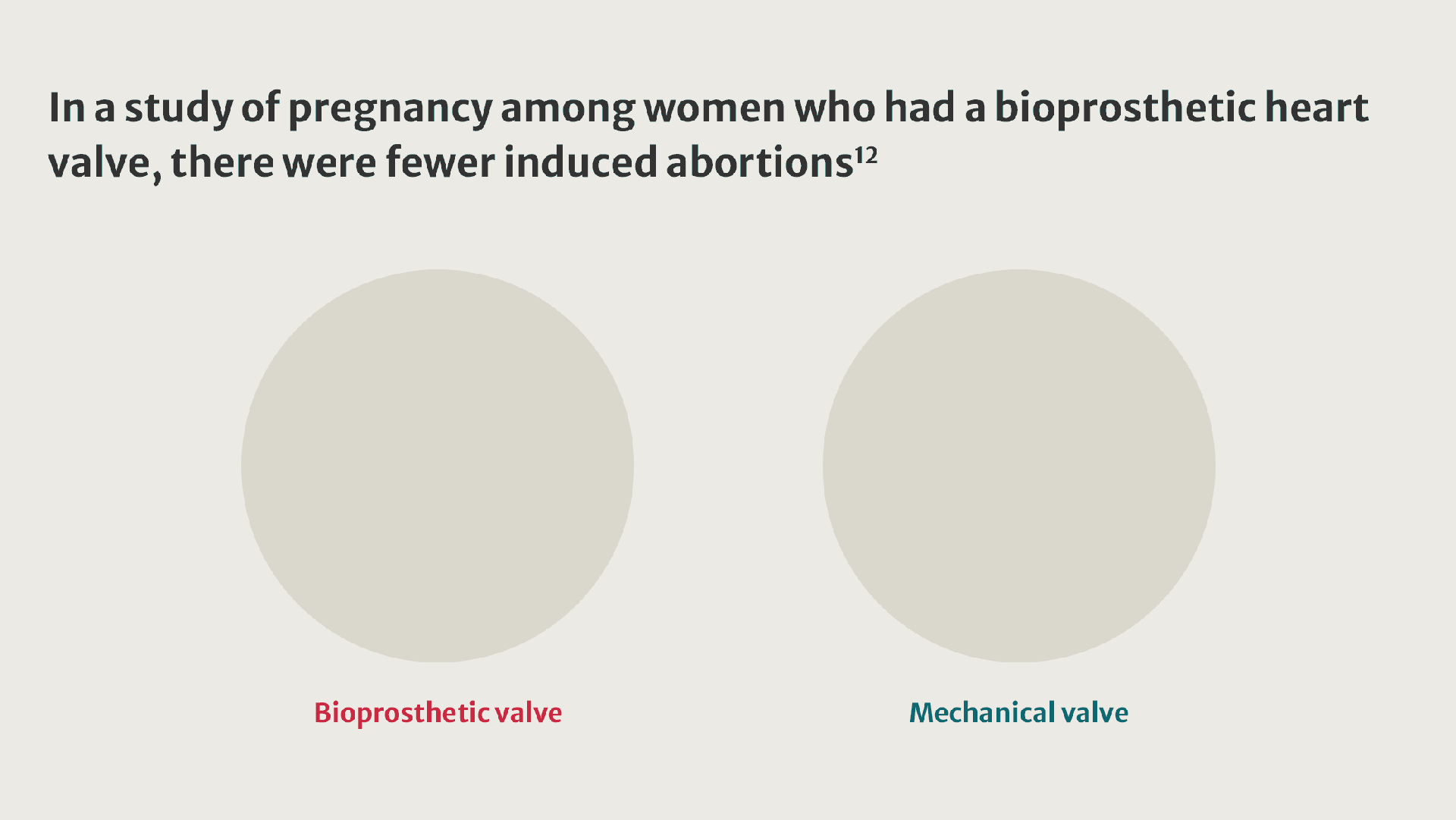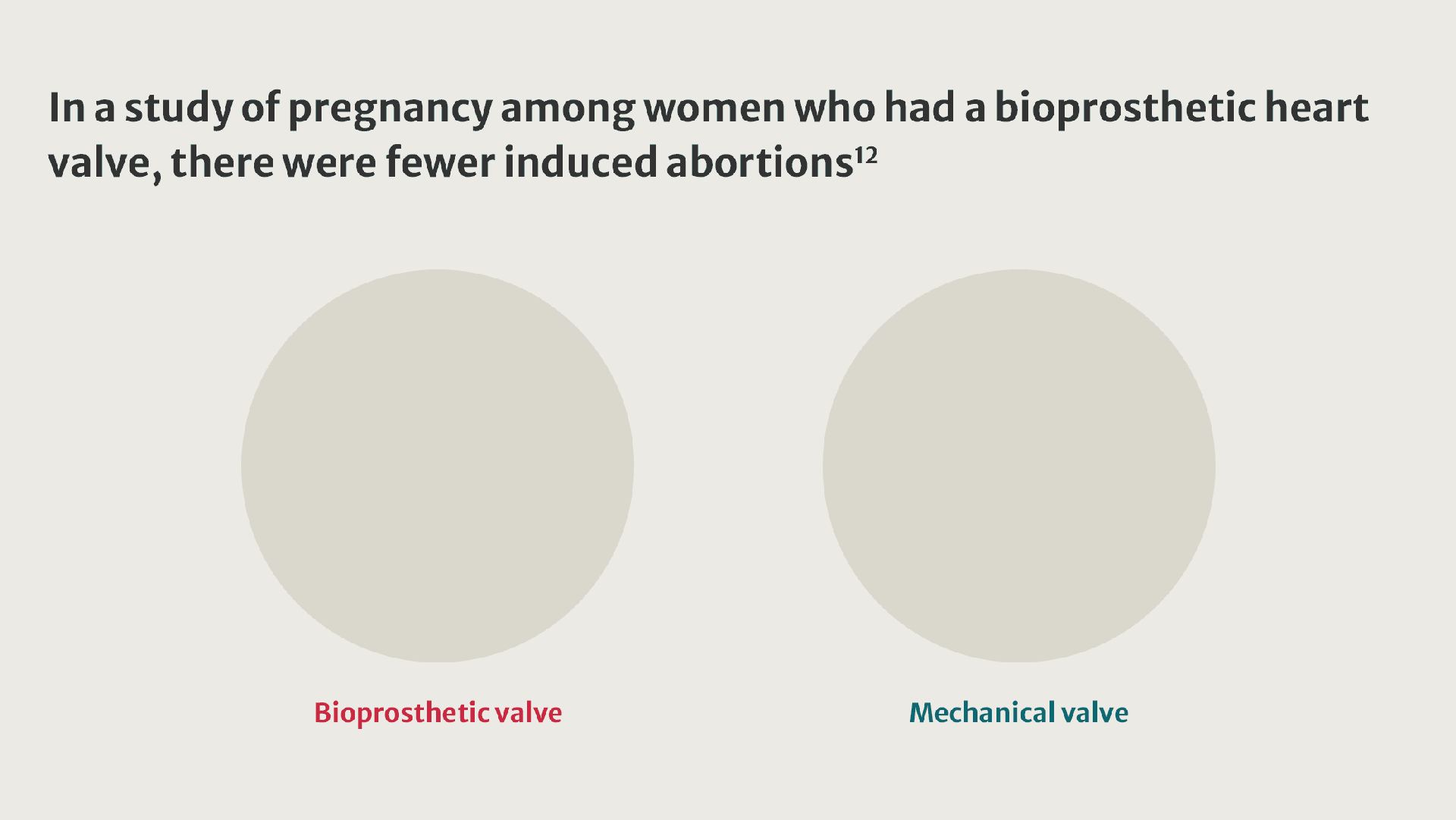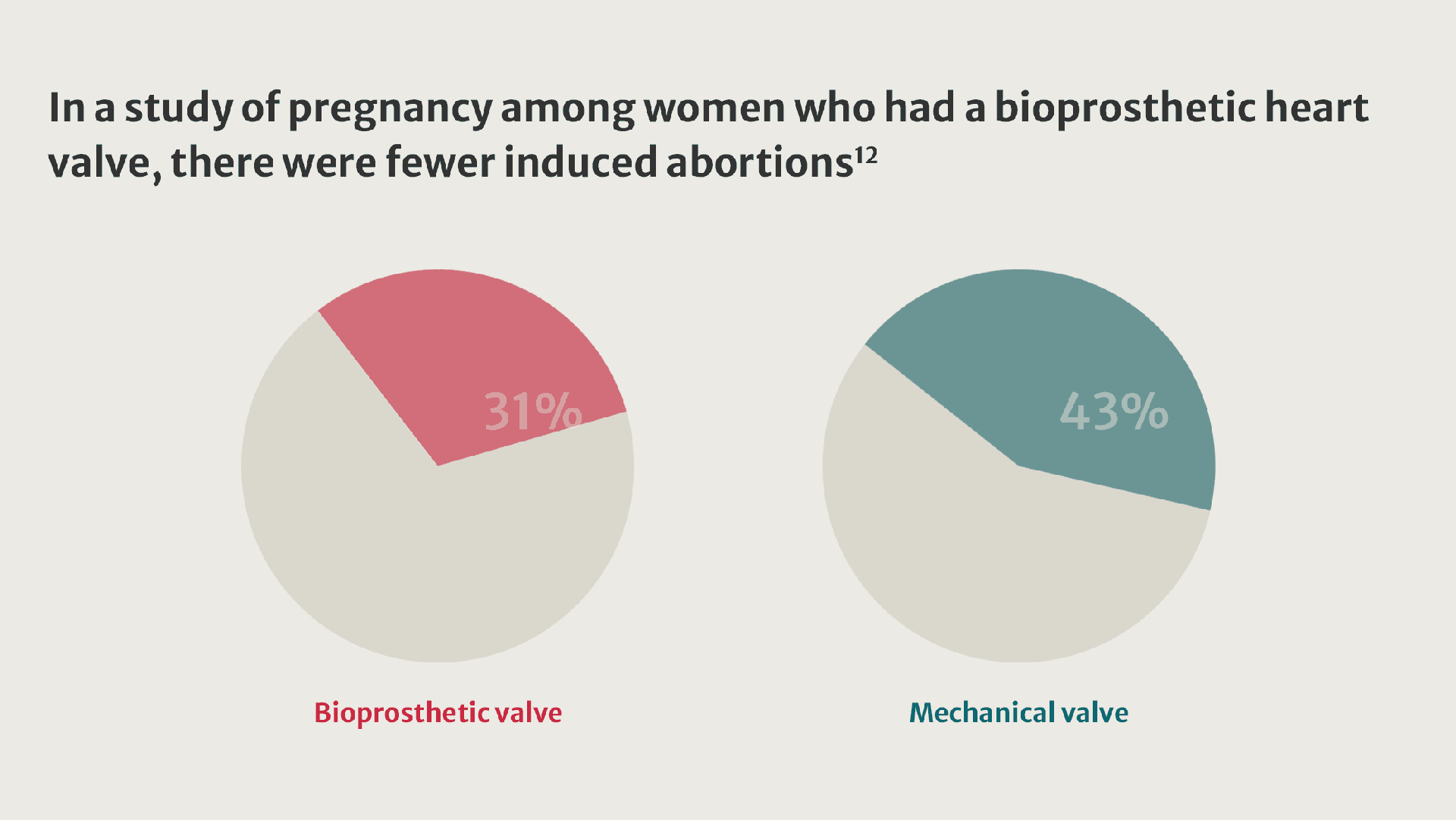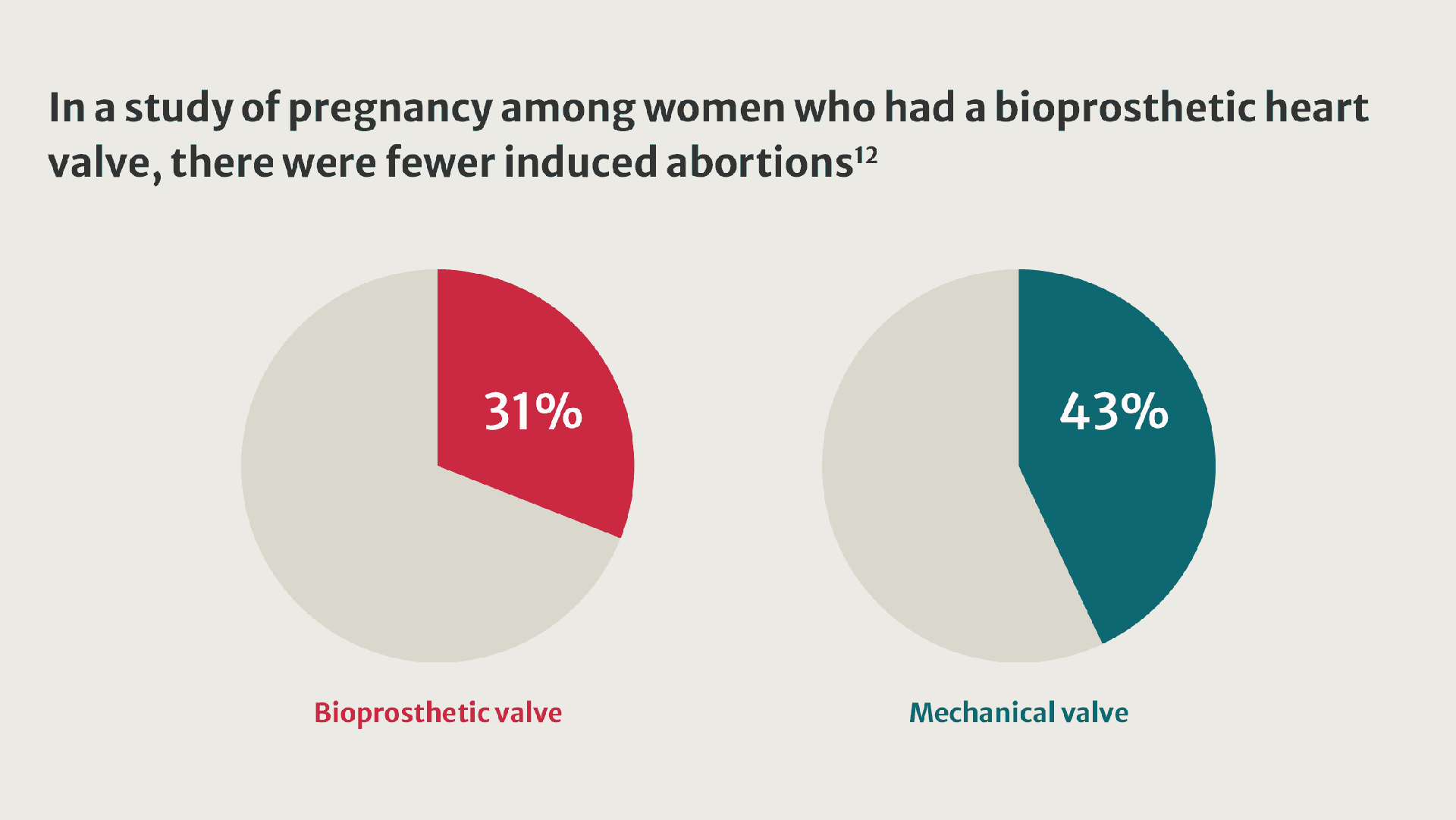
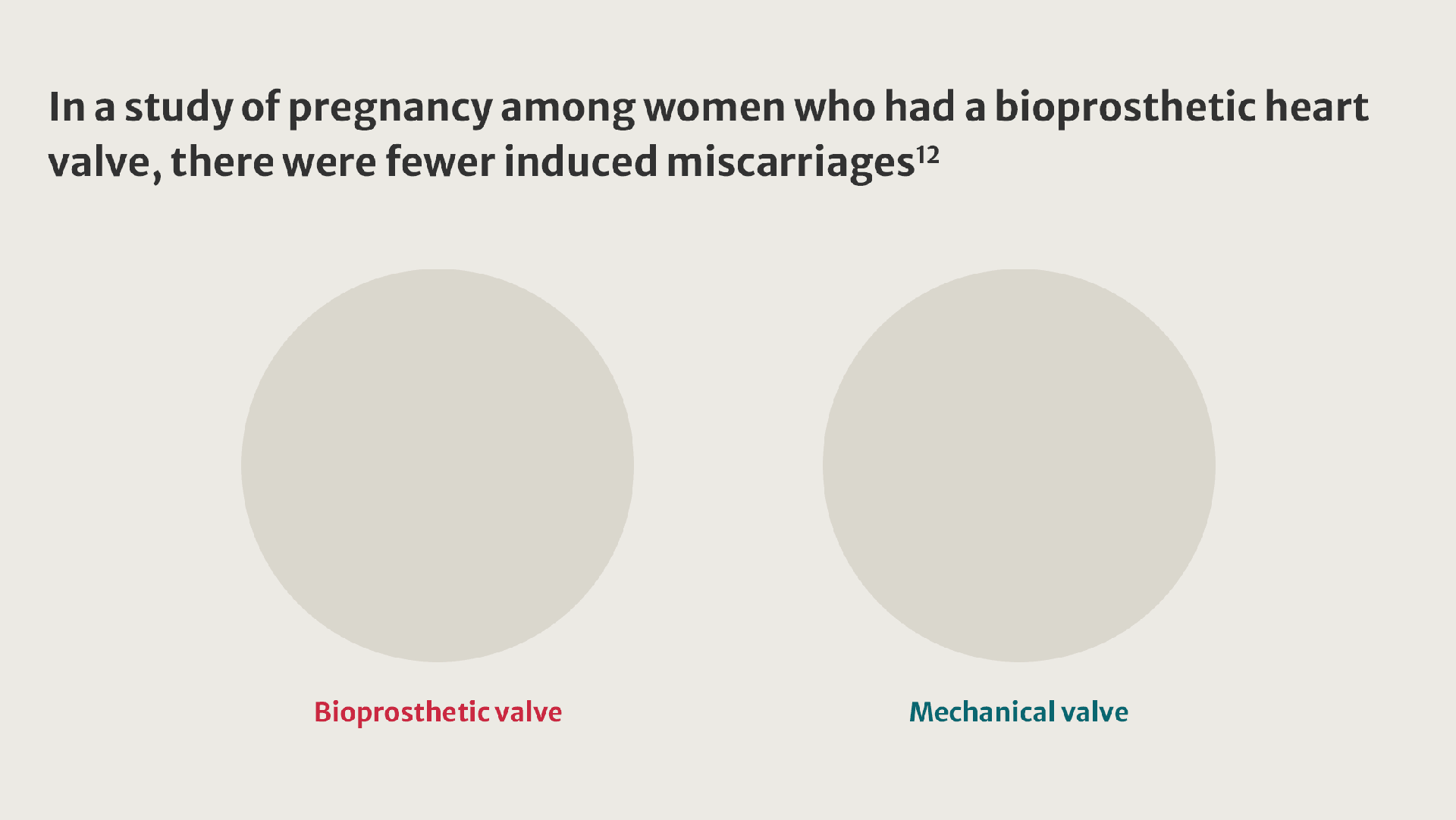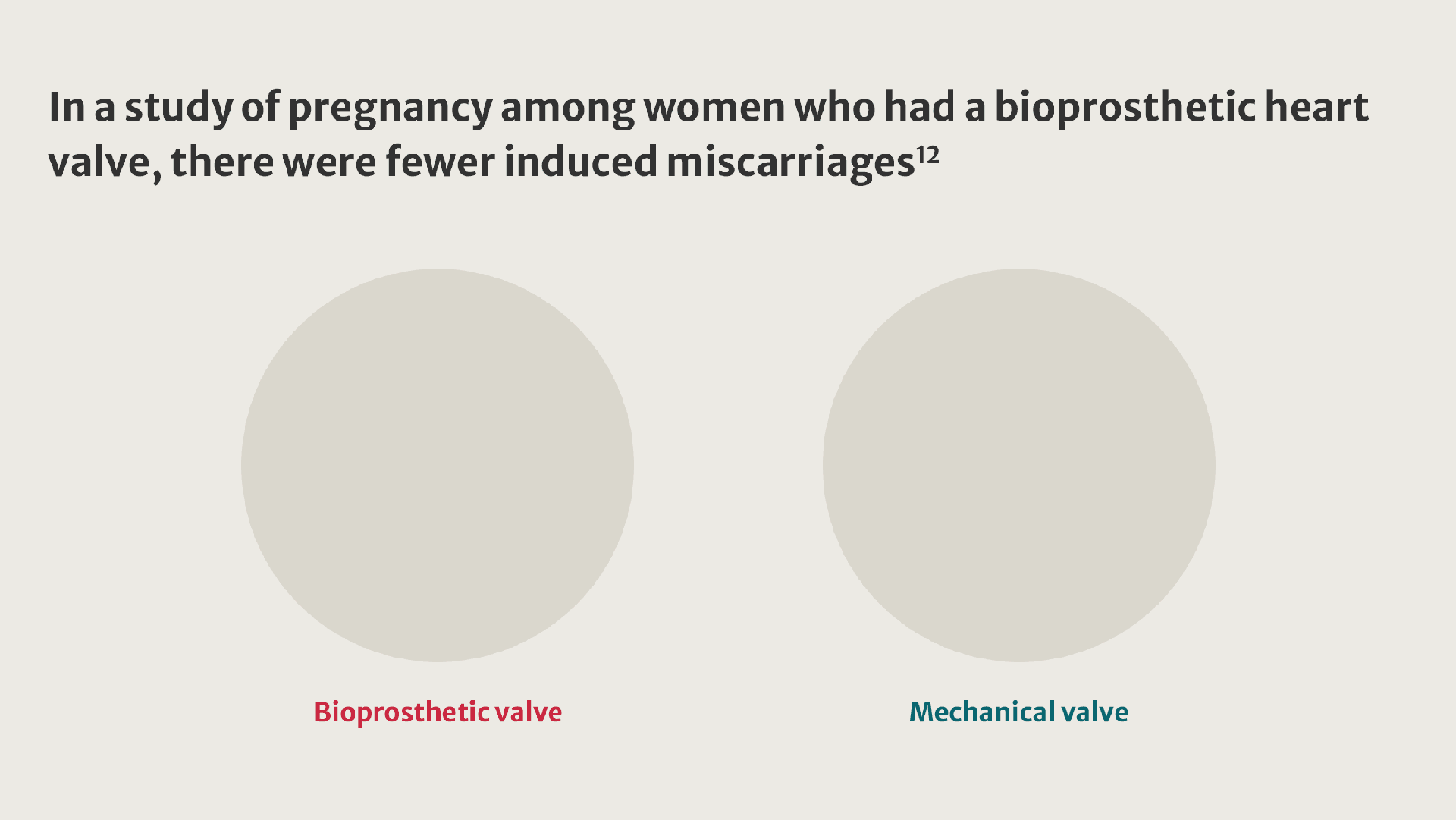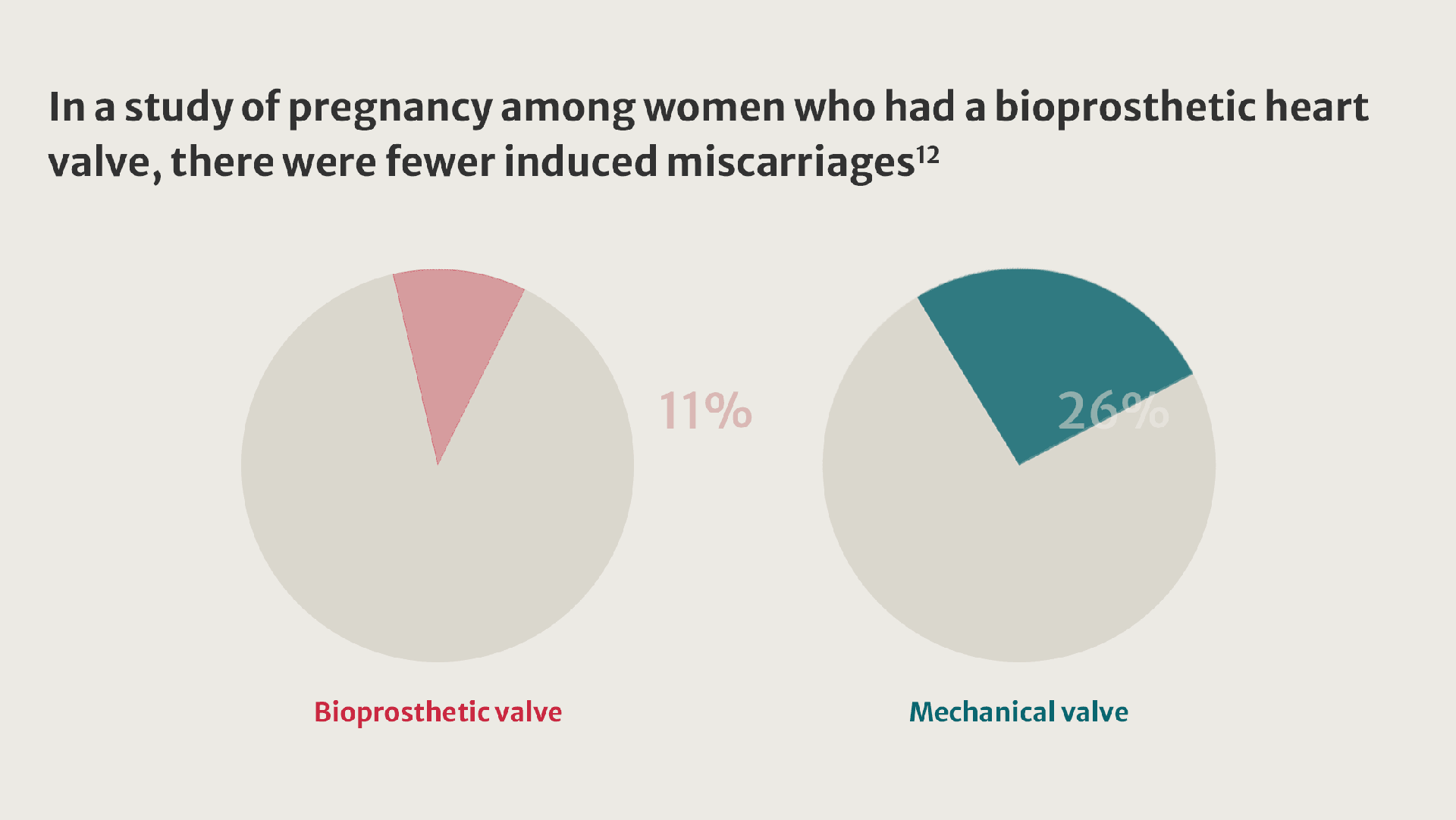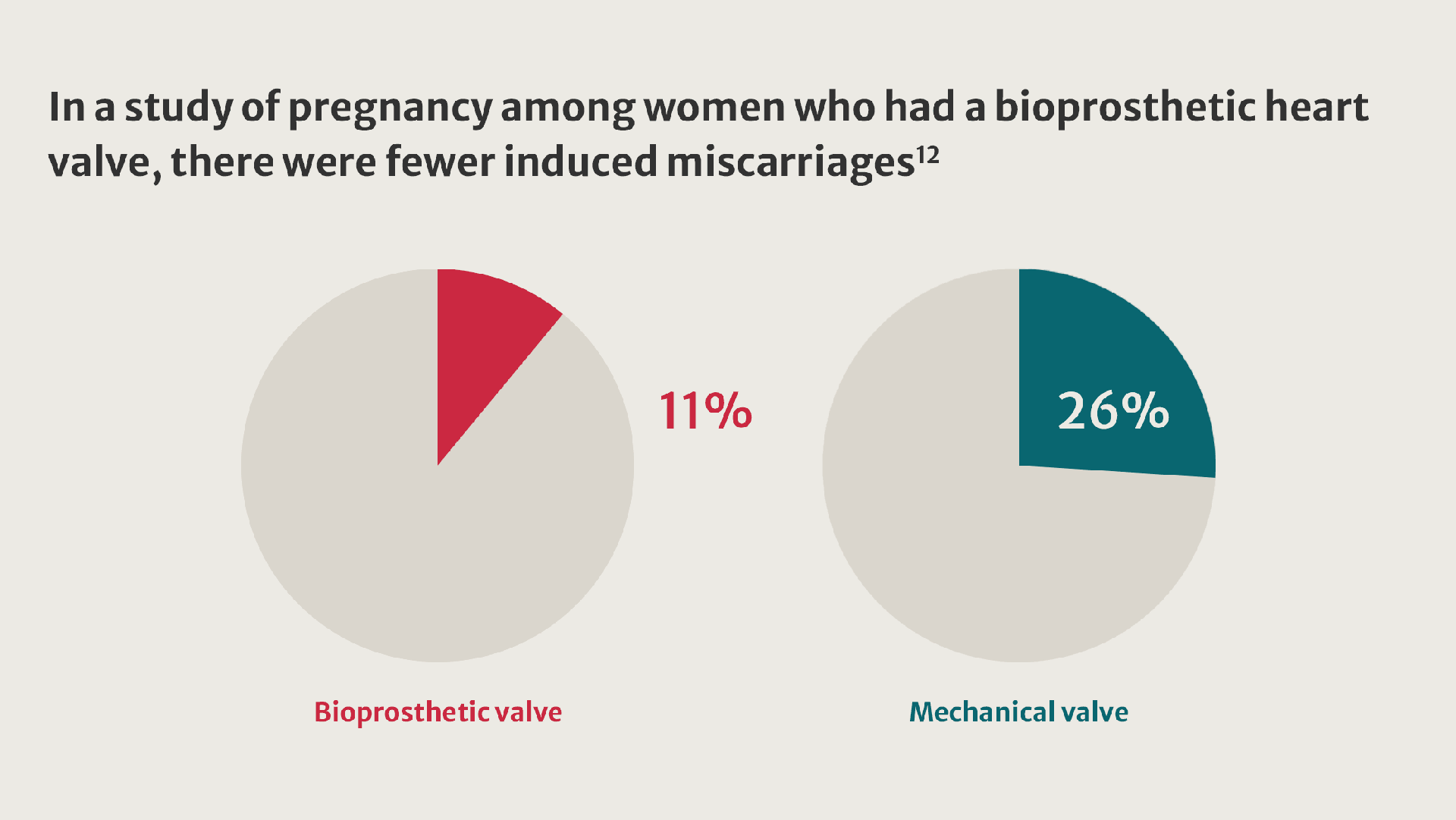
More women living with valvular heart disease are reaching childbearing age and becoming pregnant.8 Their pregnancies present challenges and are linked to an increased incidence of adverse outcomes.9
When implantation of a prosthetic valve is unavoidable in patients who want to become pregnant, valve selection can be challenging.10


Compared with women who received a tissue valve, women with mechanical valves experienced twice as many adverse events.11
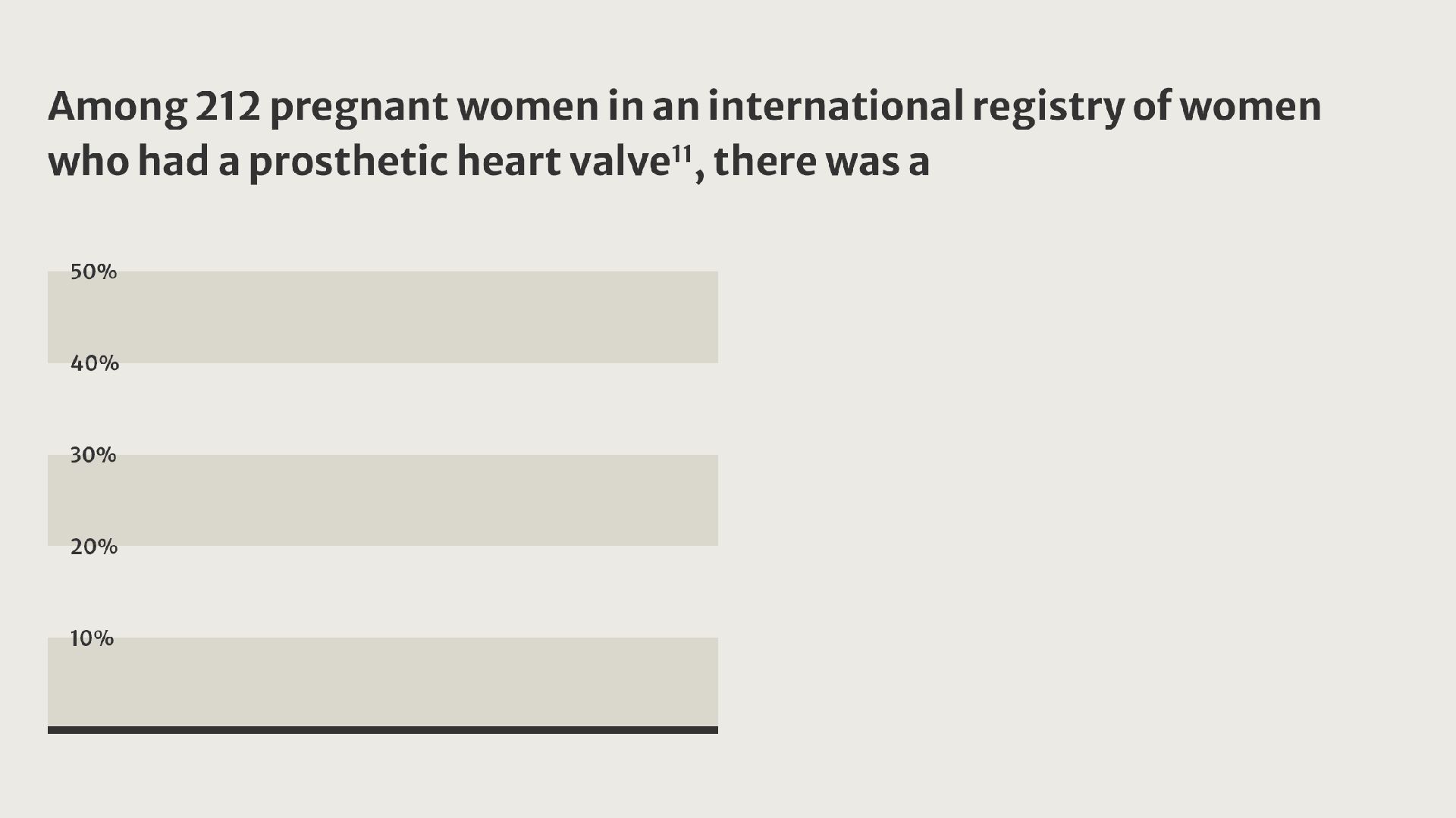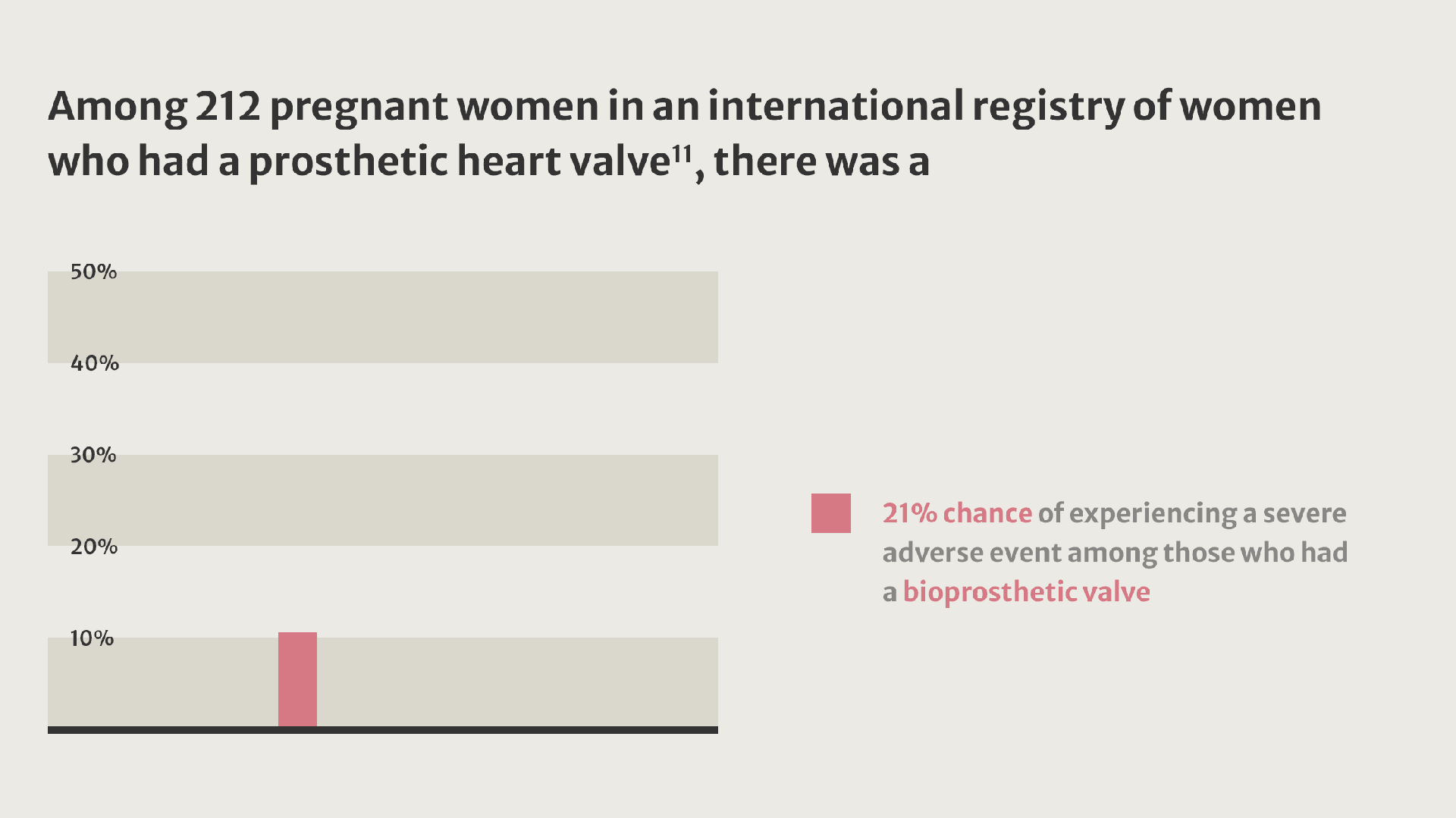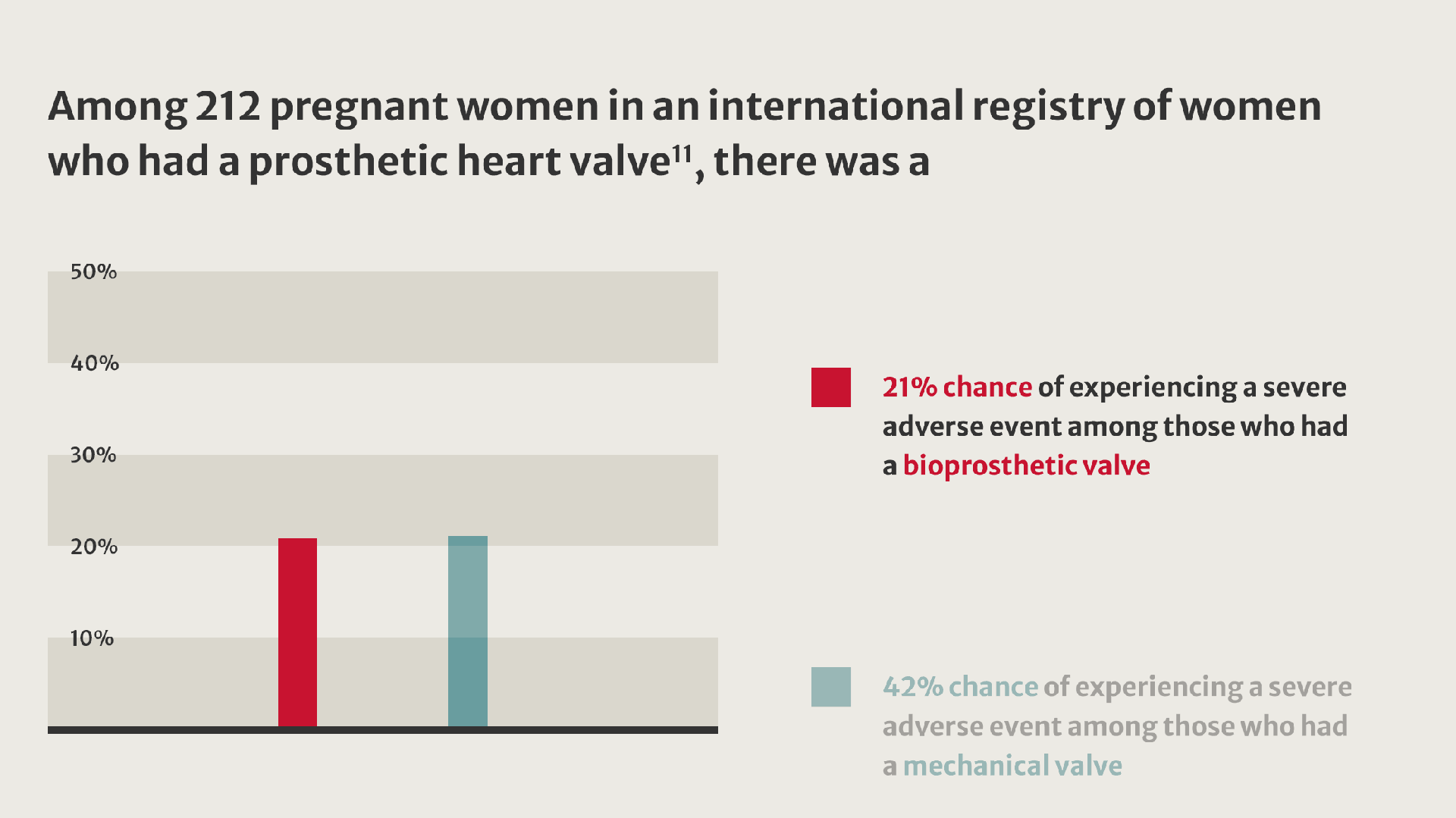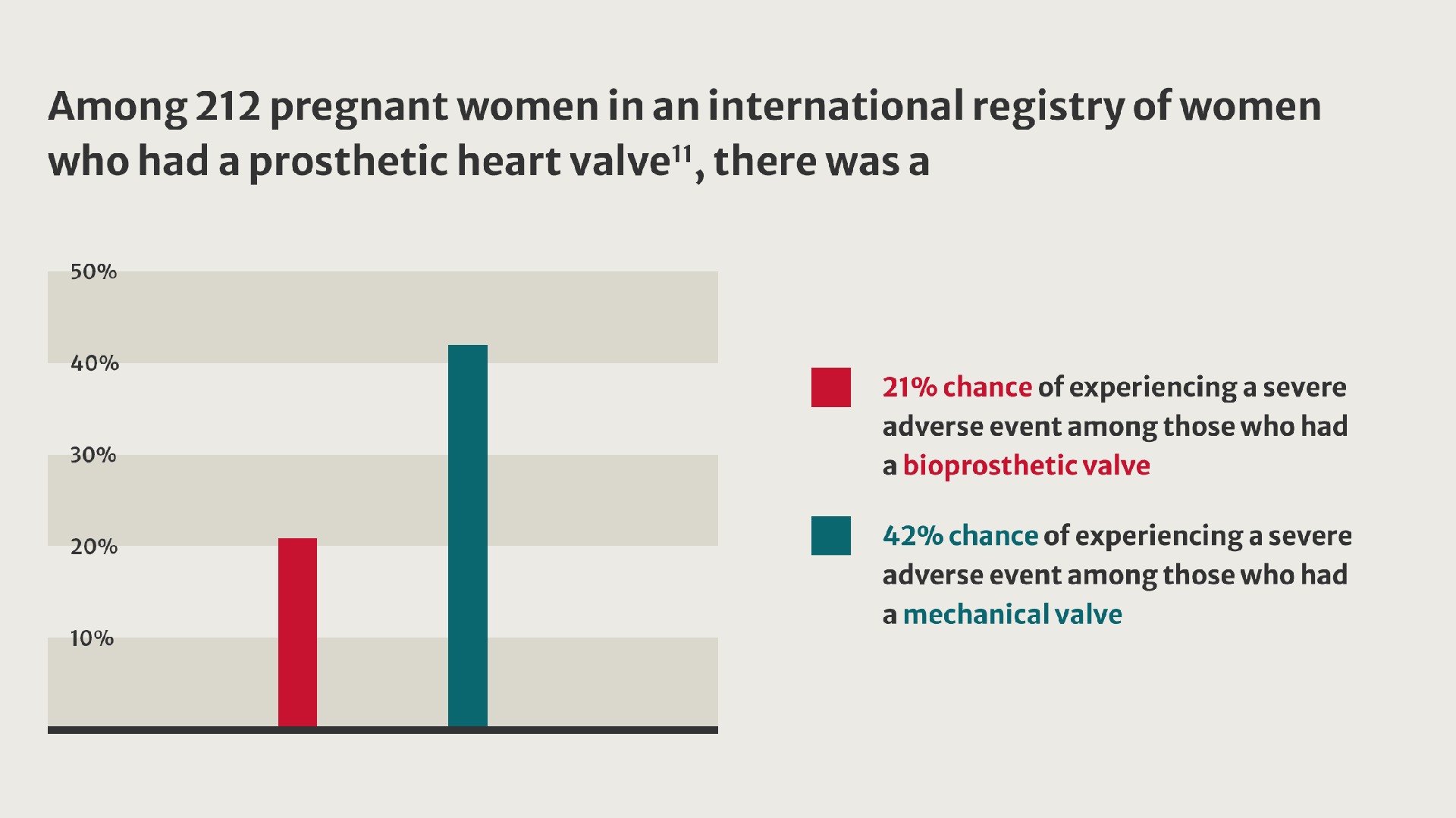

Indications: INSPIRIS RESILIA Aortic Valve - For use in replacement of native or prosthetic aortic heart valves. KONECT RESILIA Aortic Valved Conduit - For use in replacement of native or prosthetic aortic heart valves and the associated repair or replacement of a damaged or diseased ascending aorta. MITRIS RESILIA Mitral Valve - For use in replacement of native or prosthetic mitral heart valves.
Contraindications: There are no known contraindications with the use of these RESILIA tissue heart valve devices.
Complications and Side Effects: INSPIRIS RESILIA Aortic Valve - Thromboembolism, valve thrombosis, hemorrhage, hemolysis, regurgitation, endocarditis, structural valve deterioration, nonstructural dysfunction, stenosis, arrhythmia, transient ischemic attack/stroke, congestive heart failure, myocardial infarction, any of which could lead to reoperation, explantation, permanent disability, and death. Additional adverse events potentially associated with the use of polyester vascular grafts in the KONECT RESILIA AVC include hemorrhage, thrombosis, graft infection, embolism, aneurysm, pseudoaneurysm, seroma, occlusion (anastomotic intimal hyperplasia), immunological reaction to collagen (shown to be a weak immunogen; infrequent, mild, localized and self-limiting), intimal peel formation, and conduit dilatation. MITRIS RESILIA Mitral Valve - Thromboembolism, valve thrombosis, hemorrhage, hemolysis, regurgitation, endocarditis, structural valve deterioration, nonstructural dysfunction, stenosis, arrhythmia, transient ischemic attack/stroke, congestive heart failure, myocardial infarction, ventricular perforation by stent posts, any of which could lead to reoperation, explantation, permanent disability, and death.
Warnings: INSPIRIS RESILIA Aortic Valve - DO NOT ADJUST THE VALVE DIAMETER BY EXPANDING THE BAND PRIOR TO OR DURING IMPLANTATION OF THE SURGICAL VALVE. The expandable band is not designed to allow for compression or expansion during implantation of the surgical valve. This will cause damage to the valve and may result in aortic incompetence. DO NOT PERFORM STAND-ALONE BALLOON AORTIC VALVULOPLASTY PROCEDURES ON THIS VALVE FOR THE SIZES 19 – 25 mm as this may expand the valve causing aortic incompetence, coronary embolism or annular rupture. Valve-in-valve sizing in the INSPIRIS valve has only been tested with specific Edwards transcatheter heart valves. Use of other transcatheter valves may result in embolization of transcatheter devices anchored within or result in annular rupture.
CAUTION: Federal (USA) law restricts these devices to sale by or on the order of a physician. See instructions for use for full prescribing information.
How well do you know the recommendations for mechanical or tissue valves from the 2020 American College of Cardiology (ACC) /American Heart Association Guideline (AHA) Guideline for the management of valvular heart disease? Take the quiz and test your knowledge.