The Surge

The power of RESILIA tissue*†

For patients referred for surgical valve replacement, there are now three options: mechanical valves, conventional tissue valves, or RESILIA tissue valves. When it comes to quality of life, a higher proportion of patients with mechanical valves vs. tissue valves have reported dissatisfaction or uncertainty about their valve choice.1
RESILIA tissue gives surgeons the freedom to offer a resilient tissue alternative that brings the quality-of-life benefits of tissue valves to patients. That’s why Edwards Lifesciences is advancing the future of cardiac surgery with a growing portfolio of valves designed with RESILIA tissue.
RESILIA tissue offers freedom
The power of Edwards Lifesciences’ heart valves with RESILIA tissue is freedom. For surgeons, it is the freedom to focus on what matters most: patient wellbeing. RESILIA tissue offers the possibility of avoiding costly surgical re-intervention later in life when risks may be higher.
RESILIA tissue is the result of several generations of tissue valve innovation that started with the Carpentier-Edwards PERIMOUNT valve in 1981. Our continued advancement of tissue technology has led to a unique understanding of associated challenges and the best ways to address them.
A key challenge when it comes to pericardial tissue is structural valve deterioration (SVD), much of which is due to calcium buildup. RESILIA tissue is transformed to resist calcification differently.

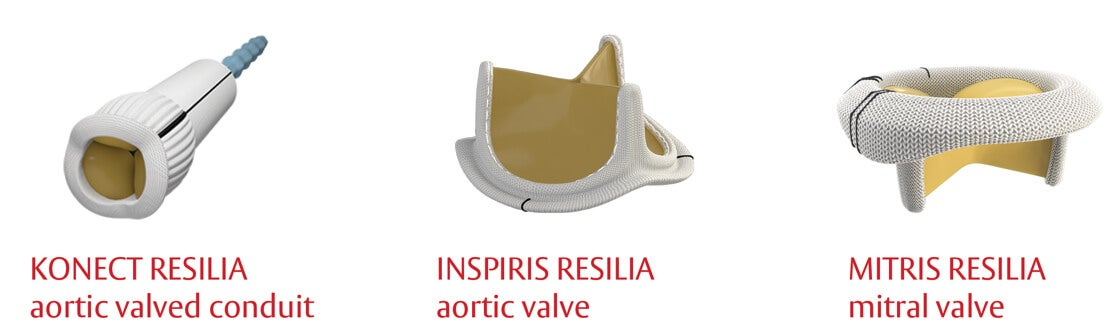
The RESILIA tissue portfolio represents the best creative scientific minds coming together to address unmet needs and satisfy patient demands for better surgical options. Currently, RESILIA tissue technology is featured on our portfolio of surgical tissue products: INSPIRIS RESILIA aortic valve, KONECT RESILIA aortic valved conduit, and MITRIS RESILIA mitral valve.
Edwards Lifesciences maintains its strong commitment to leadership in surgical heart valve development. That's why RESILIA tissue is the backbone of our future surgical heart valve development.
Learn more about how your patients can benefit from life to the power of RESILIA.
*RESILIA tissue tested against tissue from commercially available bovine pericardial valves from Edwards in a juvenile sheep model. Flameng, et al. J Thorac Cardiovasc Surg 2015;149:340–5.
†No clinical data are available that evaluate the long-term impact of RESILIA tissue in patients.
Related Articles
References
- Ruell M, Kulik A, Lam B. Long-term Outcomes of Valve Replacement with Modern Prostheses in Young Adults. European Journal of Cardio-thoracic Surgery 27 (2005) 425-433. S-10382.
Important Safety Information
RESILIA Tissue Devices
Indications: INSPIRIS RESILIA Aortic Valve - For use in replacement of native or prosthetic aortic heart valves. KONECT RESILIA Aortic Valved Conduit - For use in replacement of native or prosthetic aortic heart valves and the associated repair or replacement of a damaged or diseased ascending aorta. MITRIS RESILIA Mitral Valve - For use in replacement of native or prosthetic mitral heart valves.
Contraindications: There are no known contraindications with the use of these RESILIA tissue heart valve devices.
Complications and Side Effects: INSPIRIS RESILIA Aortic Valve - Thromboembolism, valve thrombosis, hemorrhage, hemolysis, regurgitation, endocarditis, structural valve deterioration, nonstructural dysfunction, stenosis, arrhythmia, transient ischemic attack/stroke, congestive heart failure, myocardial infarction, any of which could lead to reoperation, explantation, permanent disability, and death. Additional adverse events potentially associated with the use of polyester vascular grafts in the KONECT RESILIA AVC include hemorrhage, thrombosis, graft infection, embolism, aneurysm, pseudoaneurysm, seroma, occlusion (anastomotic intimal hyperplasia), immunological reaction to collagen (shown to be a weak immunogen; infrequent, mild, localized and self-limiting), intimal peel formation, and conduit dilatation. MITRIS RESILIA Mitral Valve - Thromboembolism, valve thrombosis, hemorrhage, hemolysis, regurgitation, endocarditis, structural valve deterioration, nonstructural dysfunction, stenosis, arrhythmia, transient ischemic attack/stroke, congestive heart failure, myocardial infarction, ventricular perforation by stent posts, any of which could lead to reoperation, explantation, permanent disability, and death.
Warnings: INSPIRIS RESILIA Aortic Valve - DO NOT ADJUST THE VALVE DIAMETER BY EXPANDING THE BAND PRIOR TO OR DURING IMPLANTATION OF THE SURGICAL VALVE. The expandable band is not designed to allow for compression or expansion during implantation of the surgical valve. This will cause damage to the valve and may result in aortic incompetence. DO NOT PERFORM STAND-ALONE BALLOON AORTIC VALVULOPLASTY PROCEDURES ON THIS VALVE FOR THE SIZES 19 – 25 mm as this may expand the valve causing aortic incompetence, coronary embolism or annular rupture. Valve-in-valve sizing in the INSPIRIS valve has only been tested with specific Edwards transcatheter heart valves. Use of other transcatheter valves may result in embolization of transcatheter devices anchored within or result in annular rupture.
Carpentier-Edwards PERIMOUNT Aortic Bioprostheses
Indications: For use in patients whose aortic valvular disease warrants replacement of their natural or previously placed prosthetic aortic valve.
Contraindications: Do not use if surgeon believes such would be contrary to the patient’s best interests.
Complications and Side Effects: Stenosis, regurgitation, endocarditis, hemolysis, thromboembolism, valve thrombosis, nonstructural dysfunction, structural valve deterioration, anemia, arrhythmia, hemorrhage, transient ischemic attack/ stroke, congestive heart failure, myocardial infarction, angina, any of which could lead to reoperation, explantation, permanent disability, and death.
CAUTION: Federal (USA) law restricts these devices to sale by or on the order of a physician. See instructions for use for full prescribing information.
Edwards, Edwards Lifesciences, the stylized E logo, Carpentier-Edwards, Carpentier-Edwards PERIMOUNT, INSPIRIS, INSPIRIS RESILIA, KONECT, KONECT RESILIA, MITRIS, MITRIS RESILIA, PERI, PERIMOUNT and RESILIA are trademarks of Edwards Lifesciences Corporation or its affiliates. All other trademarks are the property of their respective owners.
© 2022 Edwards Lifesciences Corporation. All rights reserved. PP--US-7454 v1.0
Edwards Lifesciences • One Edwards Way, Irvine CA 92614 USA • edwards.com

