The Surge

RESILIA tissue and pannus formation
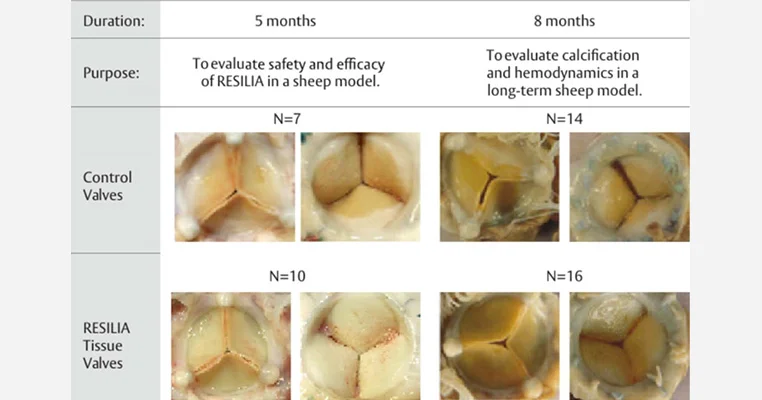
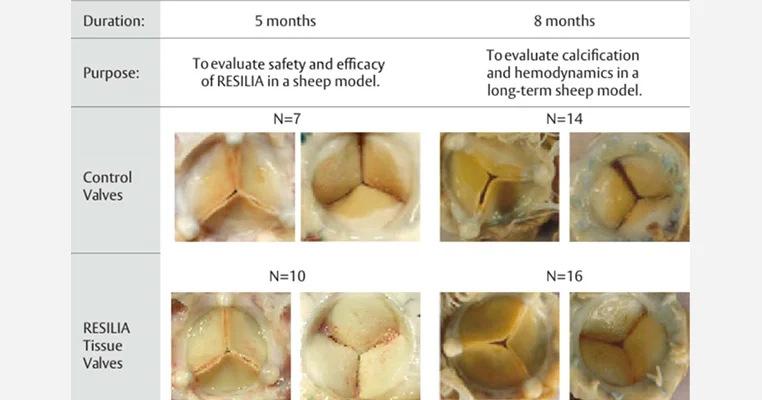
Studying the influence of resilient pericardial tissue technology on pannus formation
In the quest for improved bioprosthetic cardiac valves, much of the focus is on the leading causes of valve failure: tissue degeneration and calcification.1 There are other issues that can potentially lead to valve dysfunction, however. Pannus—a non-immune inflammatory reaction of the body to the valve prosthesis consisting of fibroelastic tissue and collagen2—can encroach on the valve leaflets, causing stenosis or obstruction.3
Designing tissue to address the causes of valve deterioration
RESILIA* tissue is bovine pericardial tissue treated with a special integrity preservation technology that effectively mitigates free aldehydes while protecting and preserving tissue. This anti-calcification technology will potentially allow the valve to last longer. The integrity preservation technology also enables dry storage by eliminating the need for a glutaraldehyde-based storage solution.
There is evidence that RESILIA tissue’s integrity preservation technology is effective in reducing calcium buildup: An earlier pre-clinical juvenile sheep model study of RESILIA tissue indicated significant improvement in anti-calcification vs. a conventional-tissue valve. At the end of 8 months, the RESILIA tissue valves had on average 72% less calcium content.4†
*No clinical data are available that evaluate the long-term impact of RESILIA tissue in patients.
†RESILIA tissue tested against tissue from commercially-available bovine pericardial valves from Edwards in a juvenile sheep model. Flameng, et al. J Thorac Cardiovasc Surg 2015;149:340-5.
Looking beyond calcification: Can the same tissue technology impact pannus formation?
To evaluate the impact of RESILIA tissue technology on pannus formation, in vivo valve performance of RESILIA tissue was studied in two different chronic sheep studies, each of which were previously published evaluating safety, calcification and hemodynamic performance. In one, an 8-month study, valves with RESILIA tissue were compared to control valves with XenoLogiX treatment (XLX). In the other, a 5-month study, valves with RESILIA tissue were compared to control valves treated with the ThermaFix process (TFX).1 Both of these control valve treatments have been used clinically for many years in commercially available valves.
The results (see Table 1 and Figures 1 and 2) show that pannus area measured over the whole RESILIA tissue valves was significantly lower than that of the control valves in both the 5- and 8-month studies compared to the control valves.
Figure 1. Pannus growth on RESILIA tissue valves compared to control valves during the 5-month study
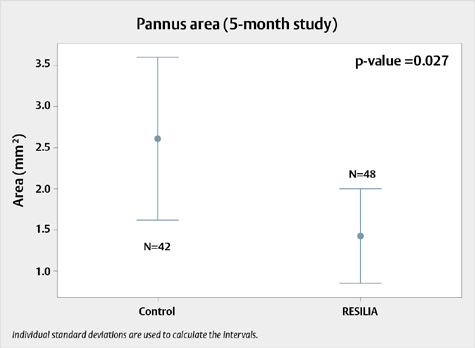
Tissue growth on RESILIA tissue valves compared to ThermaFix process control valves at 5 months. N=number of leaflets
Figure 2. Pannus growth on RESILIA tissue valves compared to control valves during the 8-month study
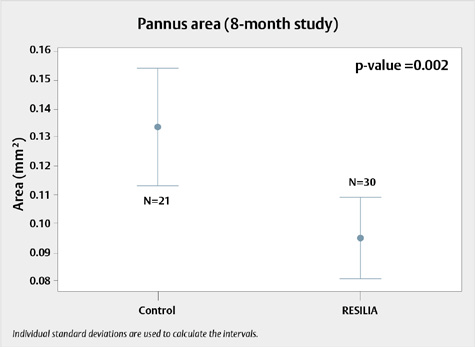
Tissue growth on RESILIA tissue valves compared to XenoLogiX treatment control valves at 8 months. N=number of leaflets
Table 1. Summary of data for the whole valve from the 5-month study
| Treatment group | Pannus area over the whole valve area (in thousands of pixels) | T-test comparison |
| Control tissue (7 valves) | 656.2 ± 385.43 | p-value = 0.010 |
| RESILIA tissue (10 valves) | 234.4 ± 265.4 | p-value = 0.010 |
- In the 5-month study, pannus area measured over the whole RESILIA tissue valves was significantly lower (234.4 ± 265.4 thousand pixels) than that of the control valves (656.2 ± 385.43 thousand pixels), with a p-value of 0.010.
- For the 5-month study, a two-sample t-test showed that the pannus on the atrial and ventricular side of each leaflet was significantly lower in test tissue samples (1.44 ± 1.52 mm2) compared to the controls (2.61 ± 2.15 mm2), with a p-value of 0.027.
- For the 8-month study, pannus measured in RESILIA tissue (0.095 ± 0.049 mm2), was significantly lower than the control tissue (0.134 ± 0.066 mm2), with a p-value of 0.002.
The 5-month study on the left shows control valves treated with TFX. The 8-month study on the right shows control valves treated with XLX. RESILIA tissue valves led to less pannus formation compared to control valves.
The authors concluded that the new tissue technology is associated with reduced pannus formation when compared to control valves, which has the potential to improve long-term outcomes for patients.1
Combined with the results of the calcification study, these new findings further support RESILIA tissue’s potential as a resilient tissue alternative to conventional pericardial tissue—and an additional option for surgeons and patients to consider.
References
1. Tod, T.J., Gohres, R.A., Torky, M. et al. Influence of Tissue Technology on Pannus Formation on Bioprosthetic Heart Valves. Cardiovasc Eng Tech 2021;12:418-25.
2. Moldovan et. al. Pannus-related prosthetic valve dysfunction. Case report. Clujul Med. 2016;89(1):169-75.
3. Darwaza, A. Recurrent pannus formation causing prosthetic aortic valve dysfunction: Is excision without valve re-replacement applicable? J Cardiothorac Surg. 2012;7:62.
4. Flameng, W., H. Hermans, E. Verbeken, and B. Meuris. A randomized assessment of an advanced tissue preservation technology in the juvenile sheep model. J. Thorac. Cardiovasc. Surg. 2015;149(1):340-45.
Important Safety Information
RESILIA Tissue Devices
Indications: INSPIRIS RESILIA Aortic Valve - For use in replacement of native or prosthetic aortic heart valves. KONECT RESILIA Aortic Valved Conduit - For use in replacement of native or prosthetic aortic heart valves and the associated repair or replacement of a damaged or diseased ascending aorta. MITRIS RESILIA Mitral Valve - For use in replacement of native or prosthetic mitral heart valves.
Contraindications: There are no known contraindications with the use of these RESILIA tissue heart valve devices.
Complications and Side Effects: INSPIRIS RESILIA Aortic Valve - Thromboembolism, valve thrombosis, hemorrhage, hemolysis, regurgitation, endocarditis, structural valve deterioration, nonstructural dysfunction, stenosis, arrhythmia, transient ischemic attack/stroke, congestive heart failure, myocardial infarction, any of which could lead to reoperation, explantation, permanent disability, and death. Additional adverse events potentially associated with the use of polyester vascular grafts in the KONECT RESILIA AVC include hemorrhage, thrombosis, graft infection, embolism, aneurysm, pseudoaneurysm, seroma, occlusion (anastomotic intimal hyperplasia), immunological reaction to collagen (shown to be a weak immunogen; infrequent, mild, localized and self-limiting), intimal peel formation, and conduit dilatation. MITRIS RESILIA Mitral Valve - Thromboembolism, valve thrombosis, hemorrhage, hemolysis, regurgitation, endocarditis, structural valve deterioration, nonstructural dysfunction, stenosis, arrhythmia, transient ischemic attack/stroke, congestive heart failure, myocardial infarction, ventricular perforation by stent posts, any of which could lead to reoperation, explantation, permanent disability, and death.
Warnings: INSPIRIS RESILIA Aortic Valve - DO NOT ADJUST THE VALVE DIAMETER BY EXPANDING THE BAND PRIOR TO OR DURING IMPLANTATION OF THE SURGICAL VALVE. The expandable band is not designed to allow for compression or expansion during implantation of the surgical valve. This will cause damage to the valve and may result in aortic incompetence. DO NOT PERFORM STAND-ALONE BALLOON AORTIC VALVULOPLASTY PROCEDURES ON THIS VALVE FOR THE SIZES 19 – 25 mm as this may expand the valve causing aortic incompetence, coronary embolism or annular rupture. Valve-in-valve sizing in the INSPIRIS valve has only been tested with specific Edwards transcatheter heart valves. Use of other transcatheter valves may result in embolization of transcatheter devices anchored within or result in annular rupture.
CAUTION: US law restricts these devices to sale by or on the order of a physician. See instructions for use for full prescribing information.
Edwards, Edwards Lifesciences, the stylized E logo, Carpentier-Edwards, INSPIRIS, INSPIRIS RESILIA, KONECT, KONECT RESILIA, PERI, PERIMOUNT, RESILIA, ThermaFix and XenoLogiX are trademarks of Edwards Lifesciences Corporation or its affiliates. All other trademarks are the property of their respective owners.
© 2022 Edwards Lifesciences Corporation. All rights reserved. PP--US-6947 v1.0
Edwards Lifesciences • One Edwards Way, Irvine CA 92614 USA • edwards.com

