The TRISCEND II trial for tricuspid regurgitation


Learn more about this clinical trial
The TRISCEND II clinical trial is to determine the safety and effectiveness of the EVOQUE valve, a device designed to replace the tricuspid valve with no open heart surgery.
This trial is for patients with severe or greater tricuspid regurgitation.

What is tricuspid valve regurgitation?1
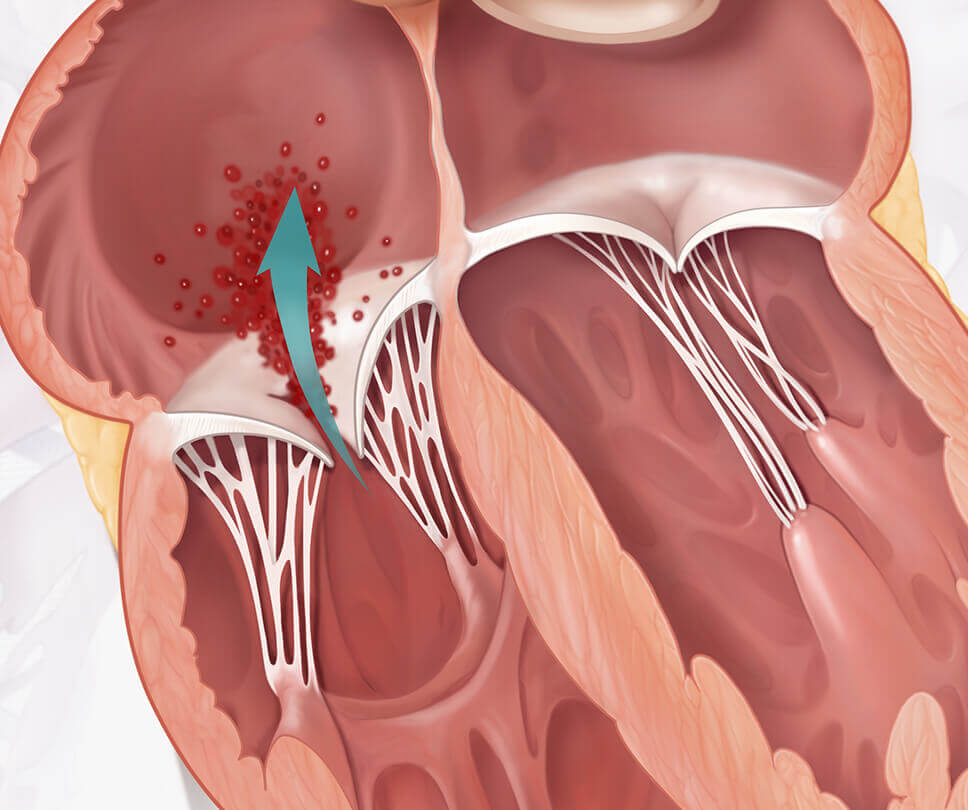
Tricuspid regurgitation (TR) occurs when the tricuspid valve in your heart does not close all the way, usually because the valve has dilated, or stretched, and its leaflets don't close tightly. This allows blood to flow backwards within the heart, and if severe may cause symptoms such as shortness of breath with activity and swelling in your abdomen, legs, or veins in your neck.


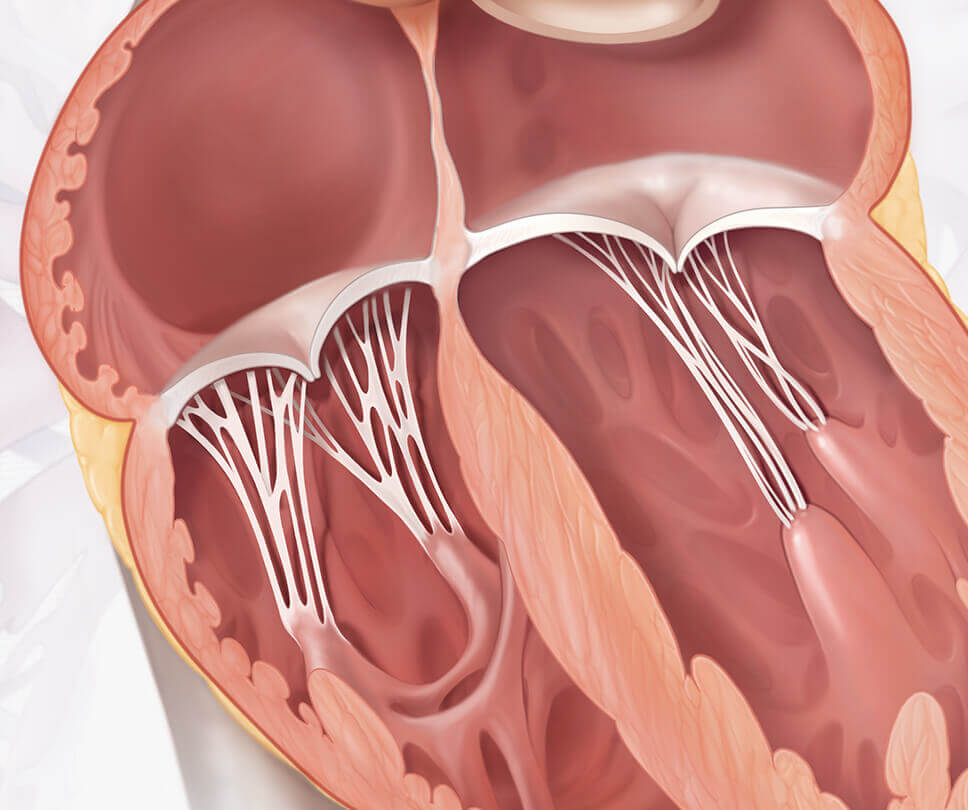
See comparison between healthy heart and one with tricuspid regurgitation
Move the arrow left or right to see comparison
EVOQUE system vs healthy heart
TR may be treated by repairing or replacing the tricuspid valve. Learn more about the TRISCEND II trial for tricuspid regurgitation treatment below.1,3
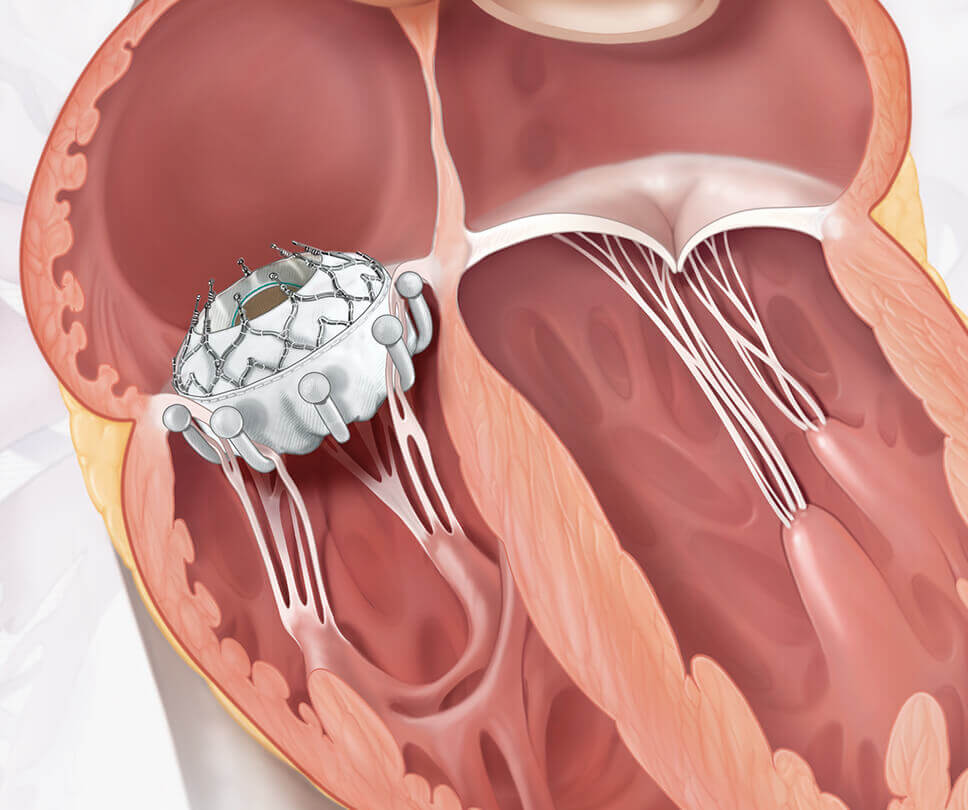
Edwards EVOQUE tricuspid valve replacement system

What is the EVOQUE tricuspid valve replacement system?
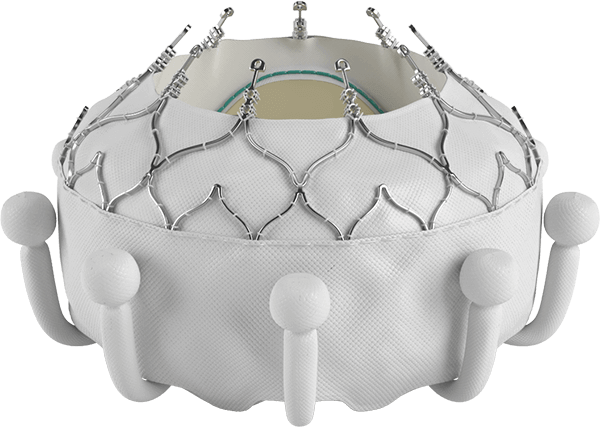

See how it works
What you should know about the TRISCEND II clinical trial
This trial is intended to evaluate the safety and effectiveness of the EVOQUE valve with Optimal Medical Therapy (OMT) compared to OMT alone in patients with severe or greater TR.2

We're here for you
We are committed to providing the highest levels of customer service to help our patients improve their quality of life. For any questions, please contact the Edwards Patient Support Center. For details about the trial, visit NCT04482062 at Clinical Trials.gov.
Give us a call
Send us an email
References
- Tricuspid valve regurgitation. Mayo Clinic. Accessed January 9, 2021. https://www.mayoclinic.org/diseases-conditions/tricuspid-valve-regurgitation/symptoms-causes/syc-20350168.
- Edwards TRISCEND II pivotal clinical trial protocol (TRISCEND II). ClinicalTrials.gov identifier: NCT04482062. Updated January 13, 2021. Accessed January 1, 2021. https://clinicaltrials.gov/ct2/show/NCT04482062.
- Clinical trials: what patients need to know. US Food and Drug Administration. Updated January 4, 2018. Accessed August 24, 2020. https://www.fda.gov/patients/ clinical-trials-what-patients-need-know.
- Inside Clinical Trials: Testing Medical Products in People. US Food and Drug Administration. Updated November 6, 2014. Accessed August 25, 2020. https://www.fda.gov/drugs/drug- information-consumers/inside-clinical-trials-testing-medical-products-people.
- Edwards EVOQUE Tricuspid Valve Replacement System: Instructions for Use. Edwards Lifesciences.
Important safety information
CAUTION – Investigational device. Limited by Federal (or United States) law to investigational use. This device is not available for marketing or commercial sale in the United States.
Edwards Lifesciences is the sponsor of the TRISCEND II trial.
Edwards, Edwards Lifesciences, the stylized E logo, EVOQUE, and TRISCEND are trademarks of Edwards Lifesciences Corporation or its affiliates. All other trademarks are the property of their respective owners
PP--US-5808 v1.0