
The PROGRESS trial


About the trial

The PROGRESS trial will examine the transcatheter aortic valve replacement (TAVR) procedure versus careful observation (or clinical surveillance) in patients who are 65 years of age or older, have moderate aortic stenosis, and have at least one additional risk factor.
By participating in this trial, you may be able to help researchers while helping others like yourself.
Contact our Patient Support Center to find a participating hospital near you
A word from the PROGRESS trial medical leaders
Why study moderate aortic stenosis?
Your heart has four valves that open and shut like doors to help pump blood throughout your body. One of the four valves is called the aortic valve. The tissue of the aortic valve’s leaflets can become stiff (due to a build-up of calcium), which causes the opening of the valve to become smaller and prevent it from opening and shutting properly. This condition is called aortic stenosis, and it affects 2.5 million people over the age of 75 in the United States.
As the opening becomes smaller, it makes it harder for the heart to pump blood, which can affect your health. Aortic stenosis is a progressive disease, meaning that the valve will get narrower and narrower, worsening over time. Slowly but surely this narrowing and increase of pressure will lead to additional cardiac problems.
Doctors will typically categorize cases of aortic stenosis as mild, moderate, or severe. The stage of aortic stenosis depends on how damaged your aortic valve is.
Disease progression in aortic stenosis
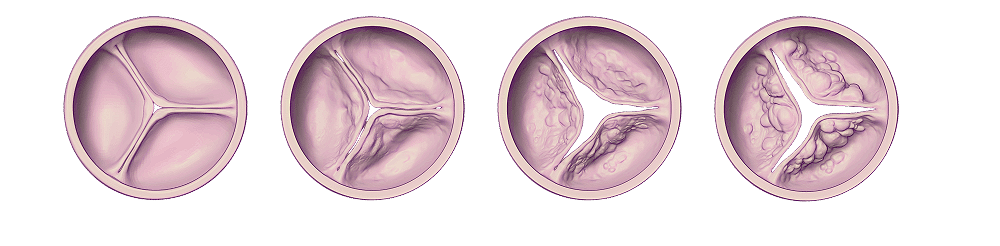
Moderate aortic stenosis is a slightly advanced stage of the disease. You may or may not notice symptoms.


Shortness of breath

Fatigue

Rapid heartbeat
What is TAVR?
Are you a candidate?
You may be eligible for this trial if you:

Talk to your doctor about the PROGRESS trial and see if it is right for you.
Ask your doctor or research coordinator for the full list of criteria. If you choose to participate, you will be asked to complete health and imaging assessments to ensure you meet the criteria to continue in the clinical trial.
What you should know about the PROGRESS trial
New medical devices are made available to people on a regular basis. However, before they can be offered to the public, they need to be studied for how safe they are and how well they work.
A clinical trial is a type of research conducted with volunteers that studies recently developed medical devices.
Questions to Ask Your Doctor

- 1Am I a candidate for the PROGRESS trial?
- 2What screening tests will be done?
- 3What is the recovery time for TAVR?
- 4What are the possible risks and benefits of the TAVR procedure?


We’re here for you
We are committed to providing the highest levels of customer service to help our patients improve their quality of life. For any questions, or if you are interested in being part of this study, please contact the Edwards Patient Support Center. For details about the trial, visit NCT04889872 at Clinical Trials.gov.
Give us a call
Send us an email
Important safety information
CAUTION: INVESTIGATIONAL DEVICES. The Edwards SAPIEN 3 and Edwards SAPIEN 3 Ultra transcatheter heart valves are investigational devices when used in patients with moderate aortic stenosis. Limited by Federal (USA) law to investigational use only. These devices are not available for marketing or commercial sale in the United States for patients with moderate aortic stenosis.
