The CLASP IID/IIF Trial for Mitral Regurgitation

CLASP IID Trial enrollment is completed.
Talk to your doctor to determine if the CLASP IIF Trial is right for you.
Get treatment for mitral regurgitation and help others like you
If you or a loved one have mitral regurgitation (MR), know you're not alone and you have options when it comes to treatment.

Learn more about this clinical trial
The CLASP IID/IIF clinical trial studies the PASCAL system, a device designed to repair the mitral valve with no open heart surgery.
This trial is meant for patients with mitral regurgitation. Talk to your doctor to determine if this trial is right for you.

What is mitral valve regurgitation?
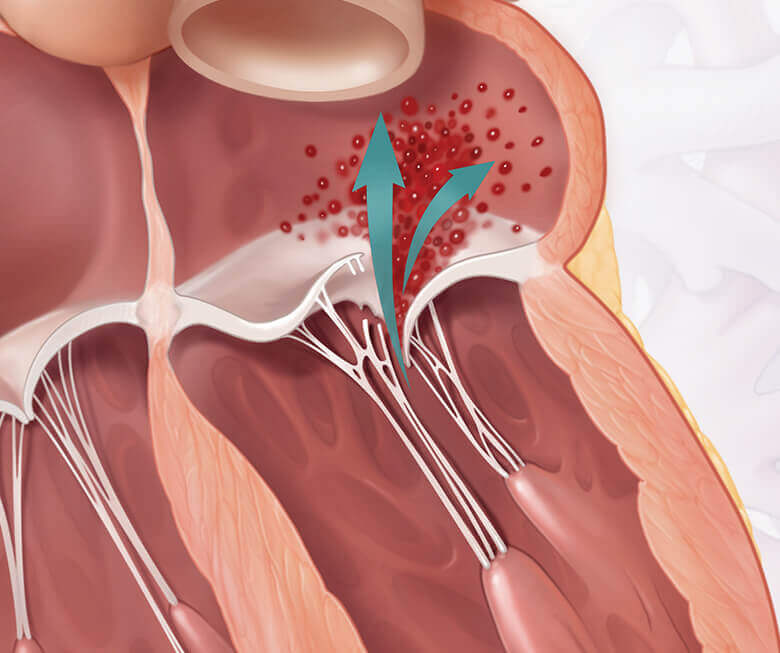
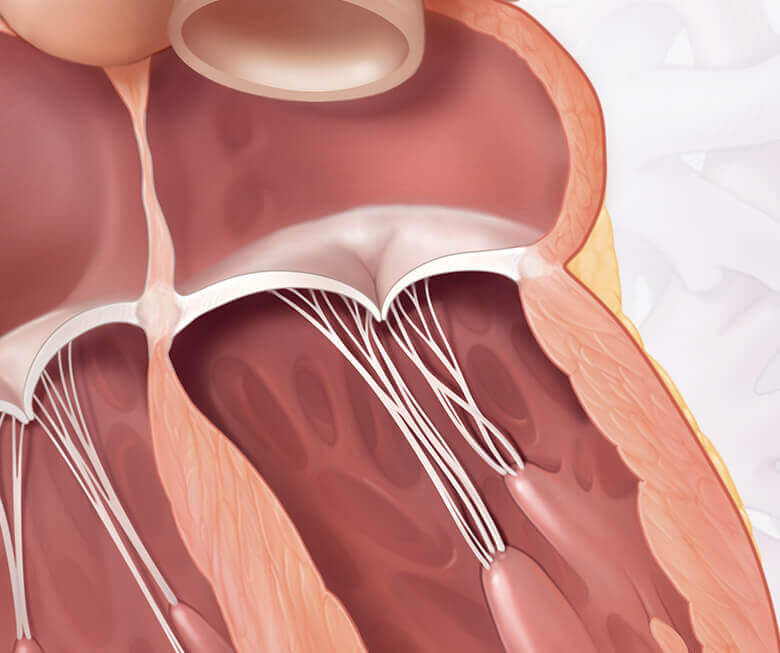
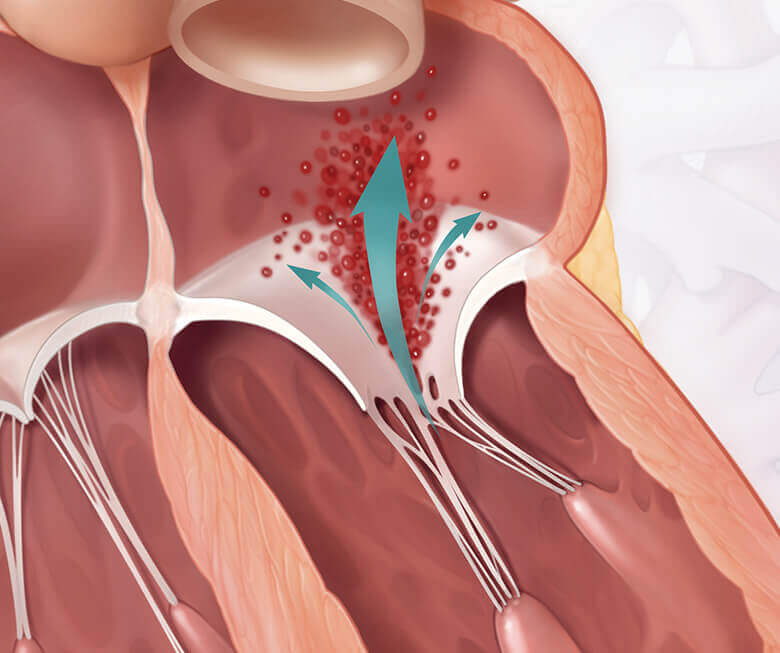
Mitral regurgitation is a condition where the heart’s mitral valve doesn’t close properly, allowing blood to flow backward within the heart. This makes it difficult for your heart to pump enough blood throughout your body. Mitral regurgitation can make you feel tired or short of breath, and can lead to heart failure if left untreated.1


Understand the two types of MR
Degenerative mitral regurgitation
Functional mitral regurgitation
Move the arrow left or right to see comparison
FMR vs healthy heart
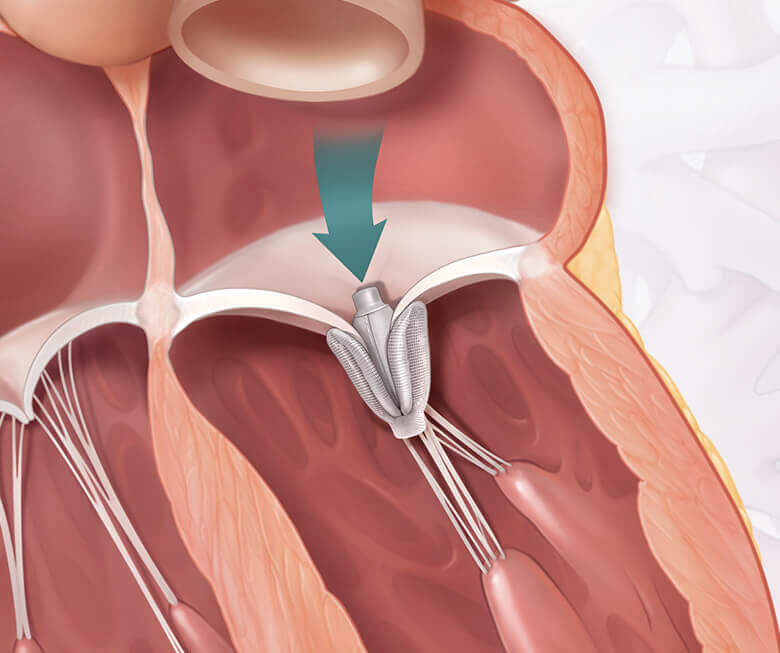
With PASCAL implant vs healthy heart
Both DMR and FMR may be treated by repairing or replacing the mitral valve. Getting treated for MR could make a difference for your heart and quality of life.1,2
The Edwards PASCAL transcatheter valve repair system

What is the PASCAL repair system?


See how it works
What you should know about the CLASP IID/IIF clinical trial
This trial is intended to evaluate the PASCAL repair system and compare it to the currently available FDA-approved device.2
Are you a candidate?
You may be eligible for the CLASP IIF Trial if you meet these criteria2:
- 1You are eighteen (18) years of age or olderand
- 2You have severe functional mitral regurgitation with stable heart failure medications as determined by your Heart Team
Talk to your doctor to see if the CLASP IIF Trial is right for you

We're here for you
We are committed to providing the highest levels of customer service to help our patients improve their quality of life. For any questions, please contact the Edwards Patient Support Center. For details about the trial, visit NCT03706833 at Clinical Trials.gov.
Give us a call
Send us an email
References
- Mitral Valve Regurgitation. Mayo Clinic. Updated Feb 08, 2022. Accessed May 26, 2023. https://mayoclinic.org/diseases-conditions/mitral-valve-regurgitation/symptoms-causes/syc-20350178.
- Edwards PASCAL CLASP IID/IIF Clinical Trial Protocol Rev E.1.
- Mitral Valve Disease: Percutaneous Interventions. Cleveland Clinic. Updated May 3, 2019. Accessed May 26, 2023. https://my.clevelandclinic.org/health/treatments/17239-mitral-valve-disease-percutaneous-interventions.
- Clinical trials: what patients need to know. US Food and Drug Administration. Updated November 6, 2014. Accessed May 26, 2023. https://www.fda.gov/patients/clinical-trials-what-patients-need-know.
- Inside Clinical Trials: Testing Products in People. US Food and Drug Administration. Updated November 6, 2014. Accessed May 26, 2023. https://www.fda.gov/drugs/drug-information-consumers/inside-clinical-trials-testing-medical-products-people.
Important safety information
Caution - Investigational device. Limited by Federal (or United States) law to investigational use. This device is not available for marketing or commercial sale for the treatment of functional mitral regurgitation in the United States. See Instructions for Use for full prescribing information, including indications, contraindications, warnings, precautions and adverse events.
Edwards Lifesciences is the sponsor of the CLASP IID/IIF trial.
Edwards, Edwards Lifesciences, the stylized E logo, and PASCAL are trademarks of Edwards Lifesciences Corporation or its affiliates. All other trademarks are the property of their respective owners
© 2023 Edwards Lifesciences Corporation. All rights reserved. PP--US-5277 v2.0