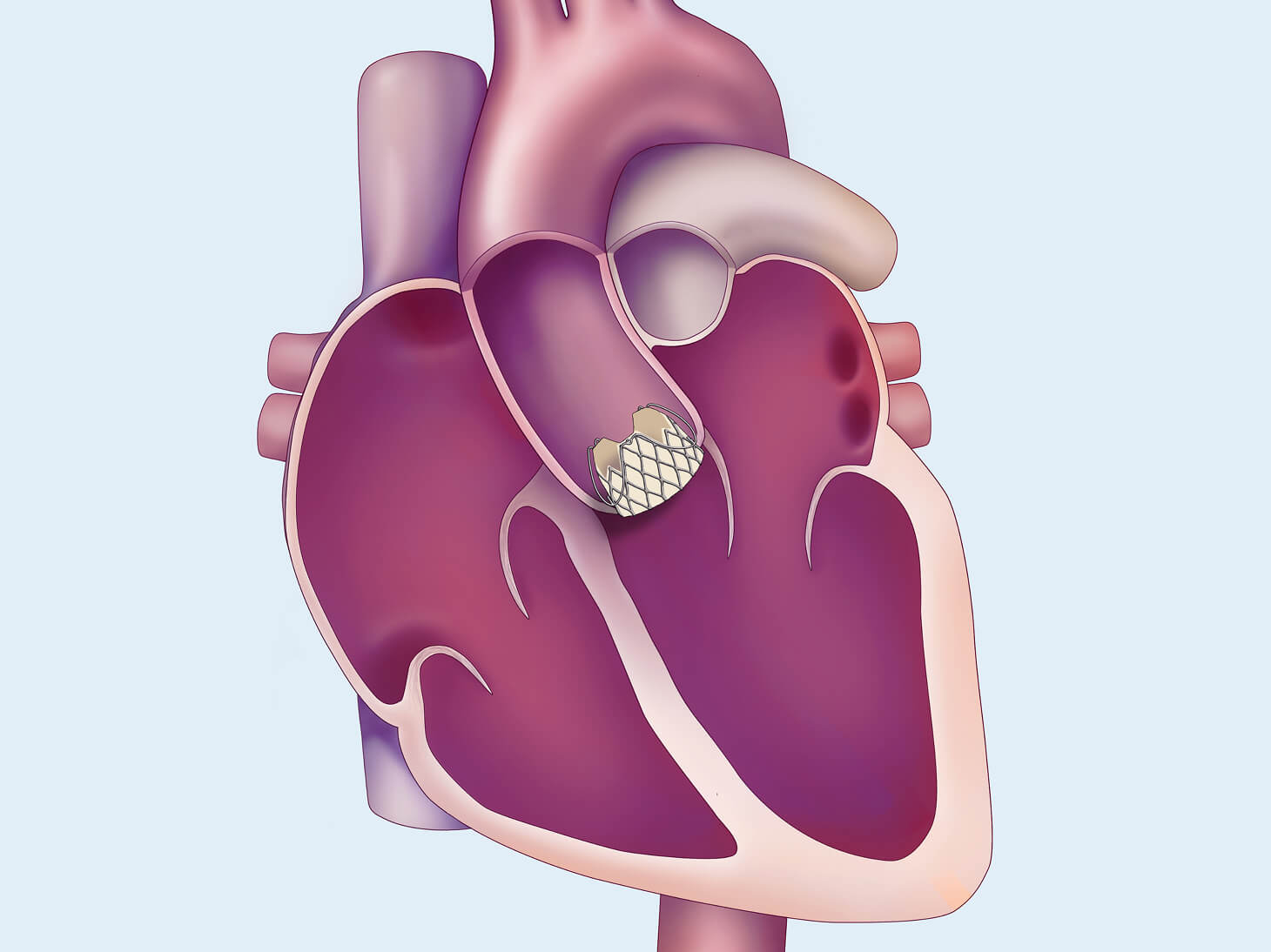
The JOURNEY trial


About the JOURNEY trial

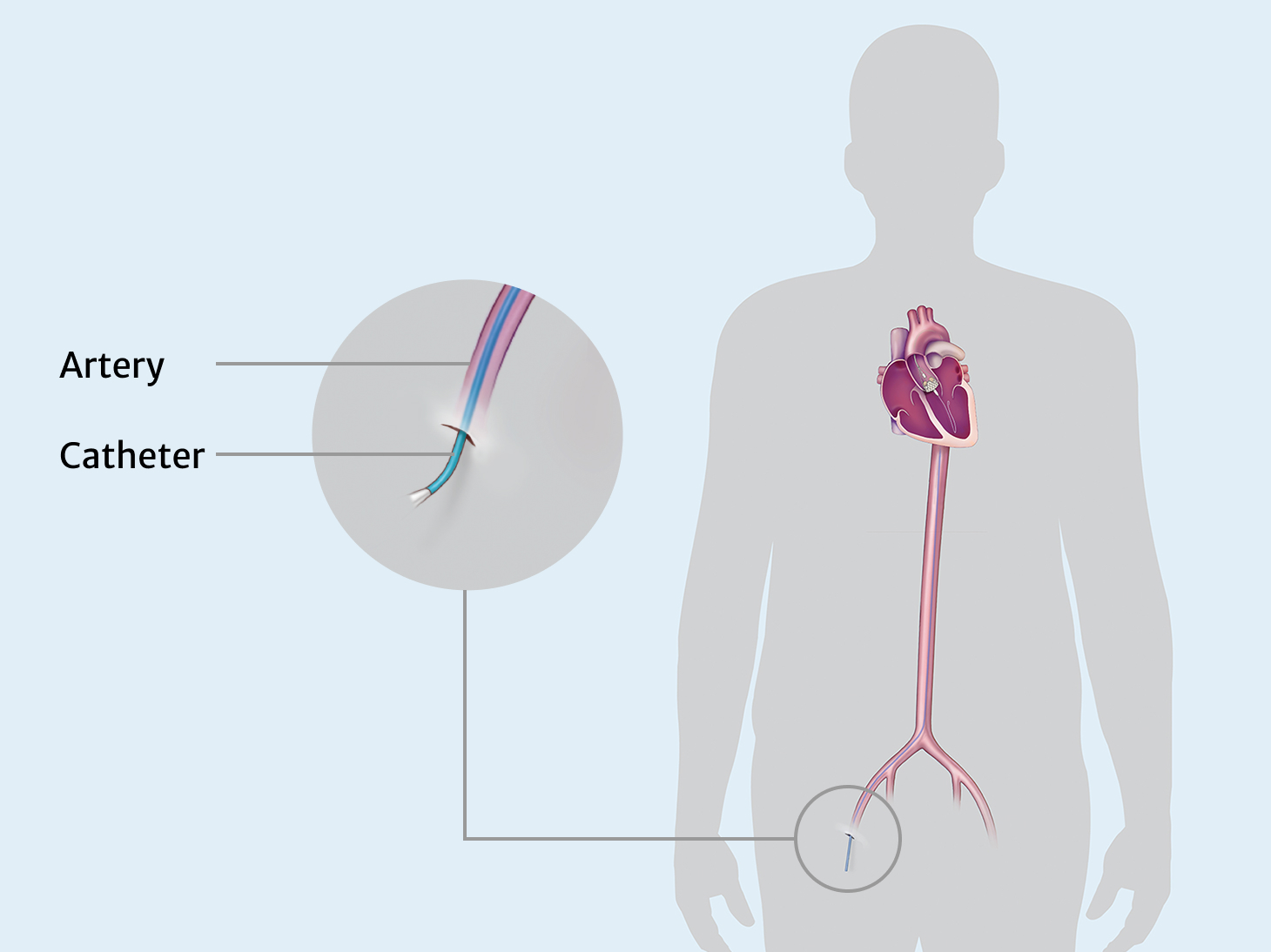
The JOURNEY trial will evaluate the safety and effectiveness of the investigational J‑Valve TF transcatheter aortic valve replacement (TAVR-AR) system for the treatment of symptomatic, severe aortic regurgitation in patients who are at high surgical risk for valve replacement by open heart surgery.
Contact our Patient Support Center to find a participating hospital near you.
What is aortic regurgitation
Aortic Regurgitation (AR) is also known as aortic insufficiency, and is diagnosed when your aortic valve allows blood to leak back into your heart instead of moving forward into your aorta.
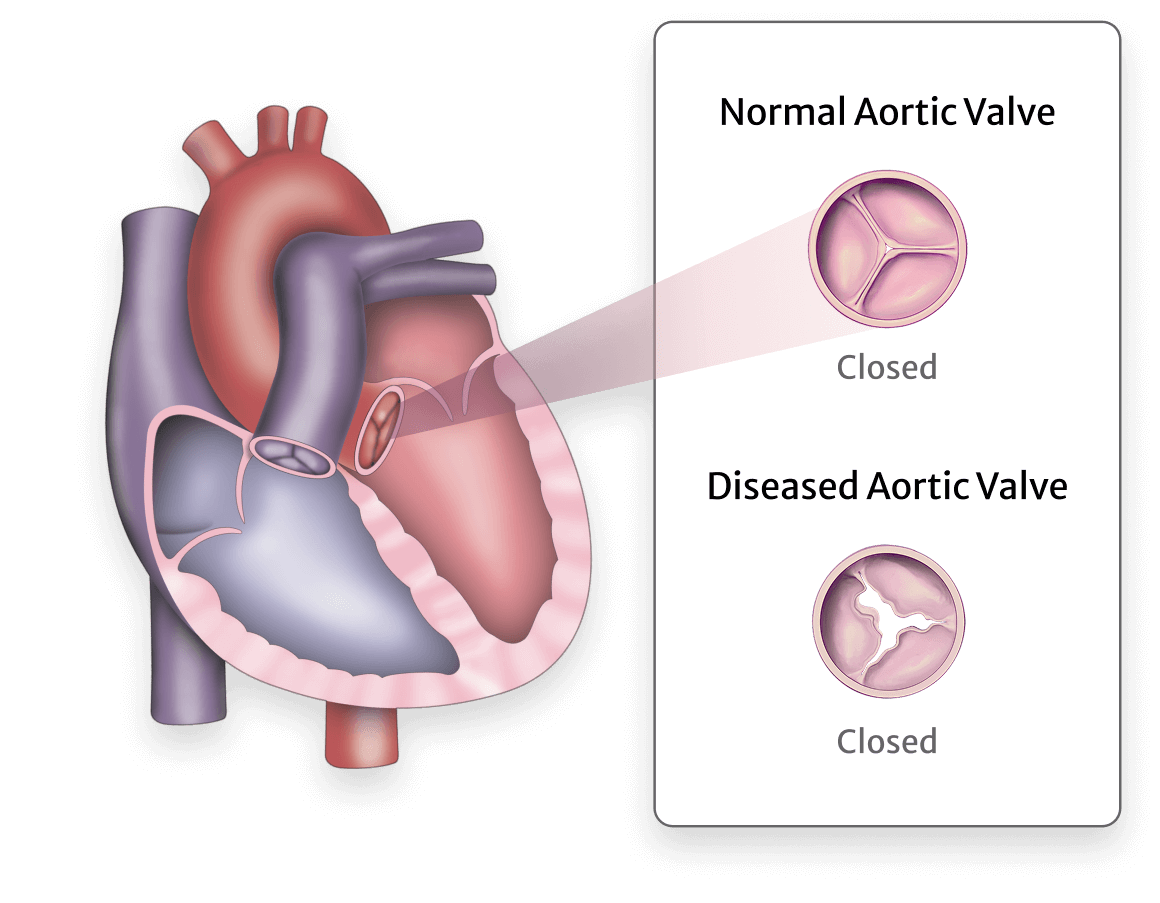
Normally, your aortic heart valve opens and closes to let blood flow from your heart's left ventricle, into your aorta, and out through your body. A leaking (or regurgitant) valve means your valve doesn't close all the way and allows the blood to flow backwards into your ventricle.
When your blood moves backwards, it forces your heart to work harder to push blood out to your body. This extra work causes your heart to grow in size (enlarge). Over time as your heart enlarges, the extra work can create symptoms and even lead to heart failure.

Symptoms can include:

Lightheadedness or fainting
Fatigue and weakness
Rapid heartbeat
Shortness of breath
Swelling of the feet
What is TAVR-AR
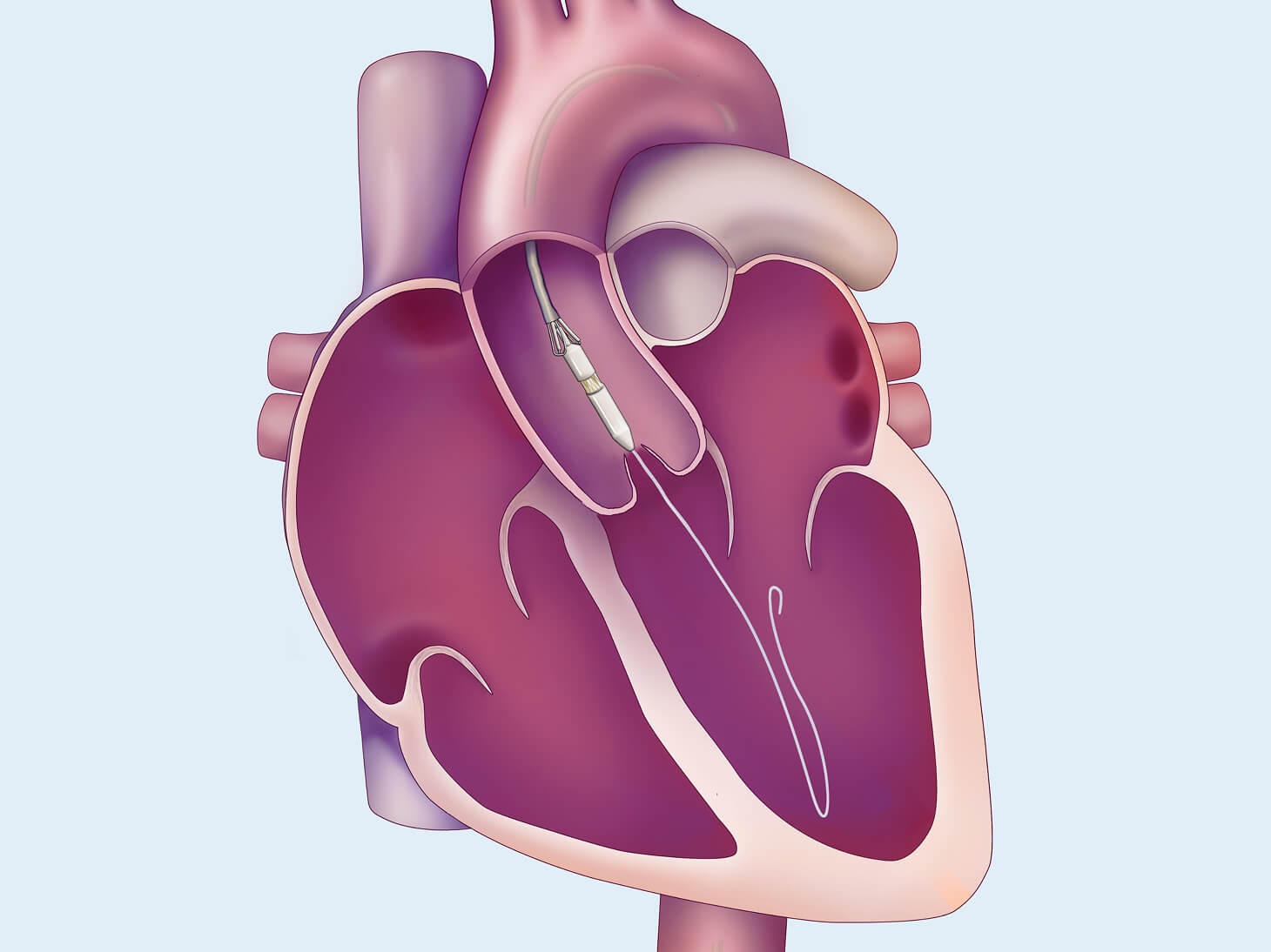
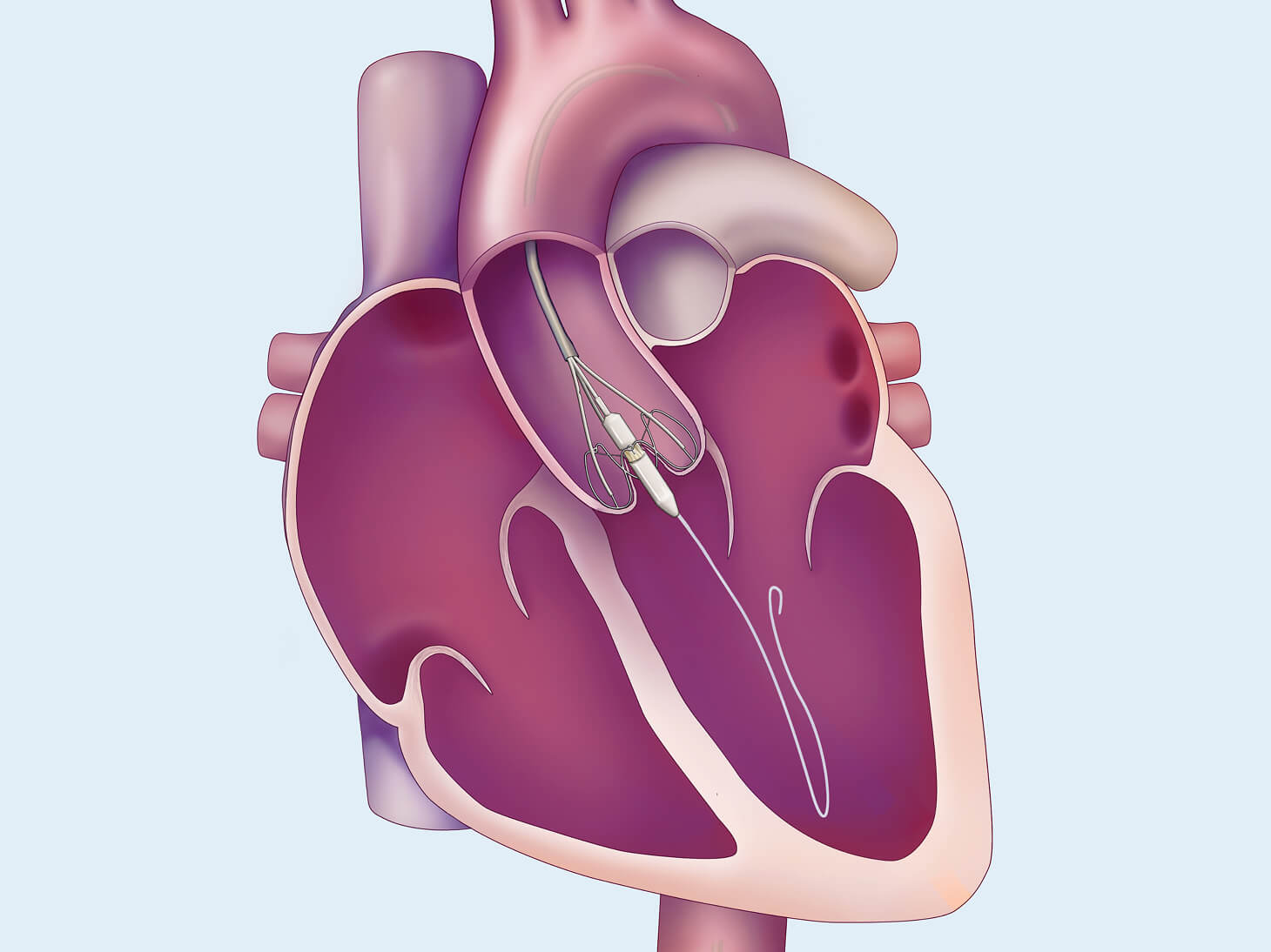
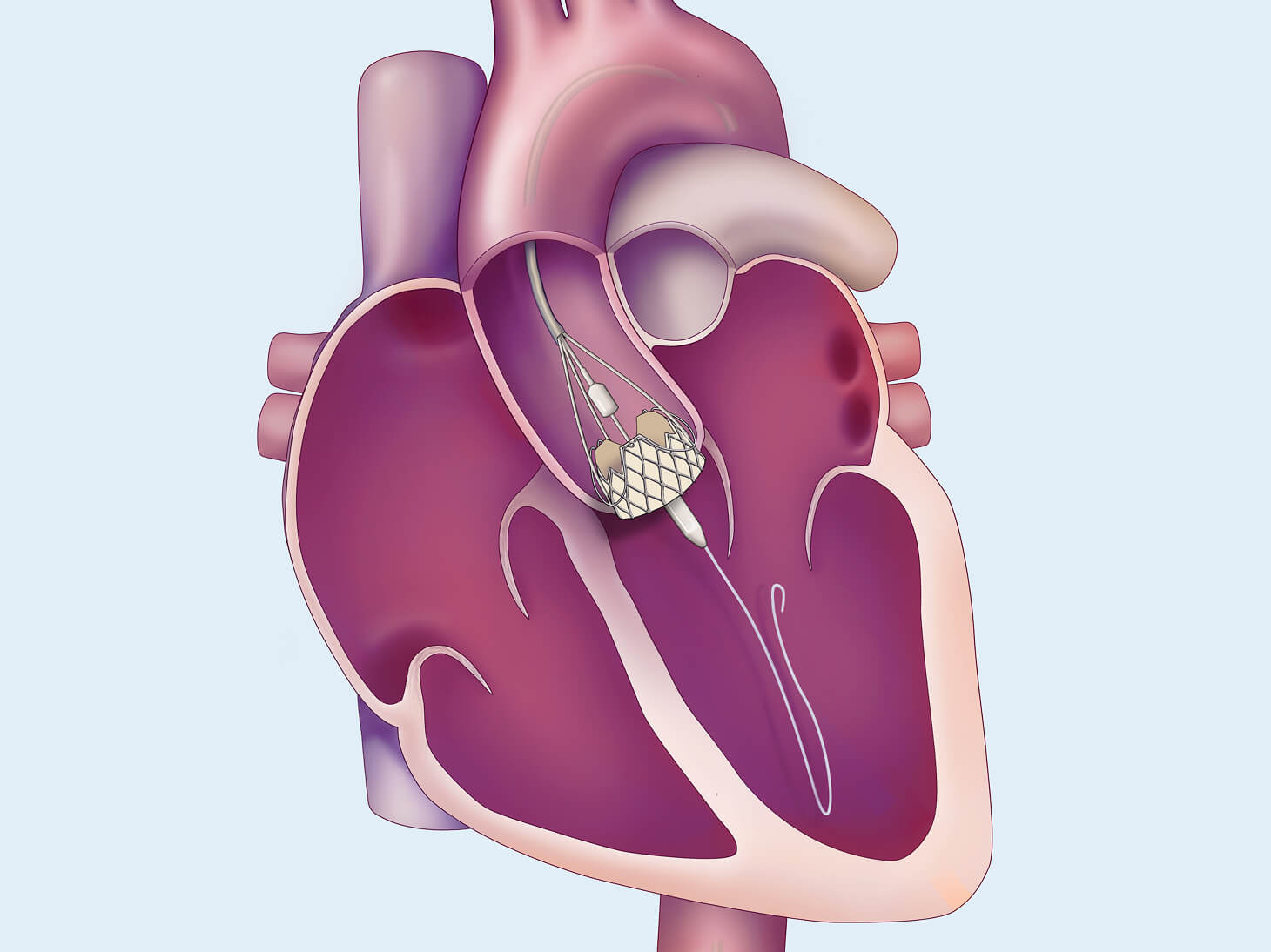
Transcatheter Aortic Valve Replacement for Aortic Regurgitation (TAVR-AR) may be a less invasive treatment for patients who are at high risk for open heart surgery. The purpose of the TAVR-AR procedure is to replace your leaking aortic valve with a bioprosthetic valve (a man-made valve containing animal tissue). We are studying the safety and performance of the investigational J-Valve device for patients with aortic valve regurgitation. This replacement is one way to treat aortic regurgitation or aortic valve insufficiency.
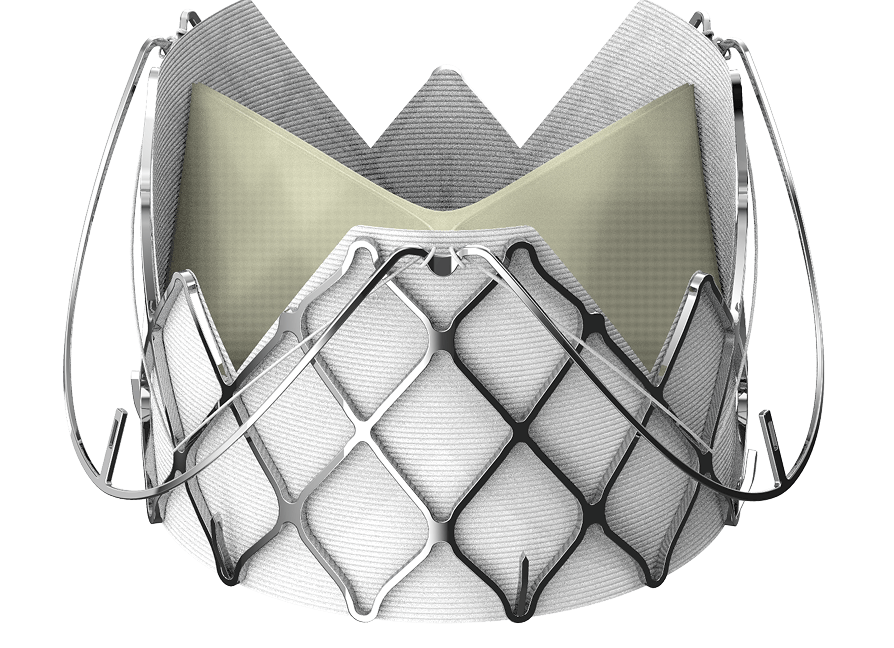
The Edwards J-Valve transcatheter heart valve

The Edwards J-Valve transcatheter heart valve
Eligibility criteria
You may be eligible for this trial if you:
- have severe, symptomatic, aortic regurgitation
- are at high risk for open surgical aortic valve replacement
Your doctor and/or research team will discuss with you the full list of criteria for participating in this trial.

Frequently asked questions

We're here for you
We are committed to providing the highest levels of customer service to help our patients improve their quality of life. For any questions, please contact the Edwards Patient Support Center.
For details about the trial, visit NCT06455787 at Clinical Trials.gov.
Give us a call
Send us an email
CAUTION: Investigational device. Limited by Federal (United States) law to investigational use.