The ENCIRCLE Trial

An Investigation of a Therapy for Severe Mitral Regurgitation
If you or a loved one suffer from mitral regurgitation (MR), you are not alone. Nearly 1 in 10 adults suffers from the disease. Learn more about the investigational ENCIRCLE trial, and you may be able to help others like yourself.


About the trial
The ENCIRCLE trial is studying the Edwards SAPIEN M3 system – a device designed to replace the mitral valve in patients with severe mitral regurgitation
This trial is meant for patients who are experiencing symptoms from their severe MR. Talk to your doctor to determine if this trial is right for you

What is mitral valve regurgitation
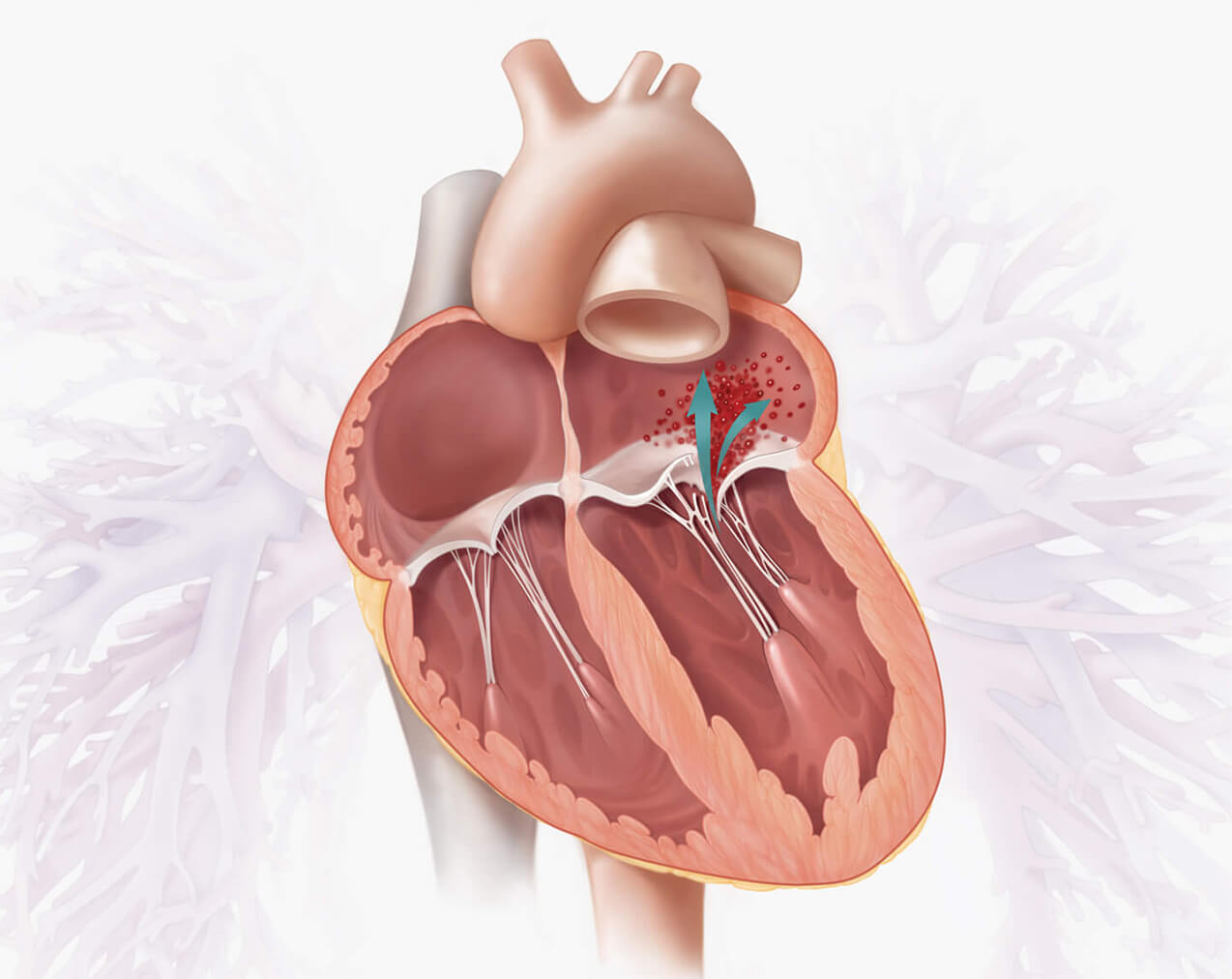
Mitral regurgitation (MR), also referred to as “leaky valve”, occurs when the mitral valve in your heart does not close all the way. This allows blood to leak backward and makes your heart work harder to pump enough blood to the rest of your body

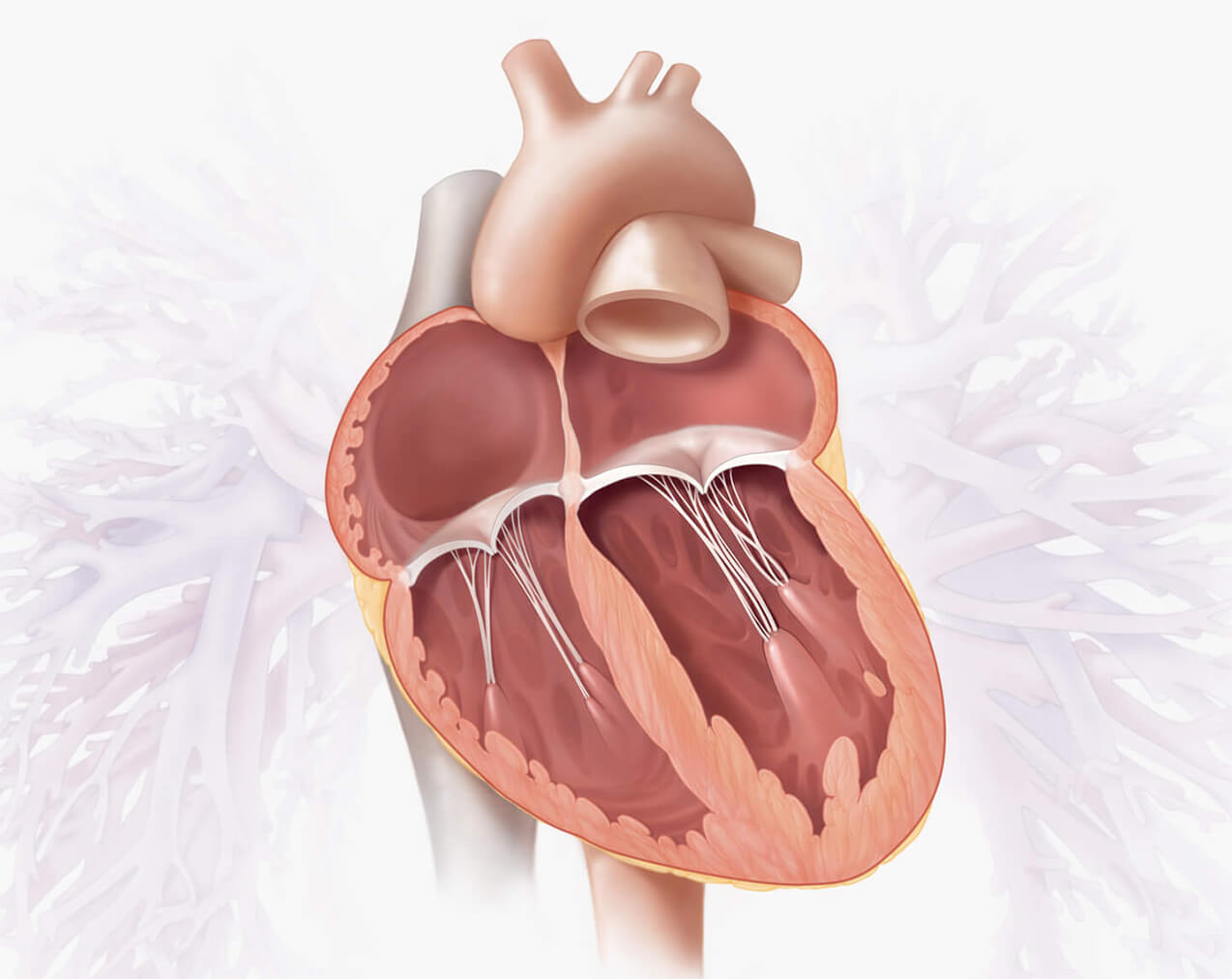
Healthy heart vs. leaking mitral valve (MR)
Healthy heart
In a healthy heart, blood flows with continuous forward motion from the right side of the heart, passing through the lungs to collect oxygen, through the left side of the heart and mitral valve, then out to the rest of your body
Diseased heart
There are a number of reasons why your mitral valve may be leaking: torn valve tissue, damage to the valve itself, and stretching due to an enlarged heart chamber are some of the possible reasons
Symptoms can include:


Inability to exercise

Fluid build up in the lower body

Feeling tired
Typically, MR becomes more severe over time as your heart gets weaker from working harder, and if untreated can lead to heart failure or even death
What you should know about the ENCIRCLE trial
Learn more about the ENCIRCLE trial procedure
The Edwards SAPIEN M3 System
The SAPIEN M3 system is designed to replace your mitral valve through a minimally invasive procedure called transcatheter mitral valve replacement

- The SAPIEN M3 dock is designed to encircle the native mitral anatomy to provide a suitable anchoring location for the SAPIEN M3 valve
- The SAPIEN M3 valve has been adapted for use in the mitral position from the SAPIEN 3 valve
Are you a candidate?
You may be eligible for this trial if you meet these key criteria:
- Your mitral valve isn’t working properly and leaking
- You are experiencing symptoms of your MR
- Your doctor determines any prior mitral interventions you may have had or attempted do not impact this new valve from working properly
Talk to your doctor about possible risks and benefits, and see if this trial is right for you
Ask your doctor or research coordinator for the full list of criteria, risks, and benefits. If you choose to participate, you will be asked to complete health and imaging assessments to ensure you meet the criteria to continue in the clinical trial.


We're here for you
We are committed to providing the highest levels of customer service to help our patients improve their quality of life. For any questions, please contact the Edwards Patient Support Center. For details about the trial, visit NCT04153292 at ClinicalTrials.gov.
Give us a call
Send us an email
Caution: Investigational device
Limited by Federal (United States) law to investigational use.
Intended for US audiences only.
© 2021 Edwards Lifesciences Corporation. All rights reserved.
Edwards Lifesciences ∙ One Edwards Way, Irvine CA 92614 USA