
The EARLY TAVR Trial


About the trial

The EARLY TAVR trial is no longer enrolling patients. The EARLY TAVR Trial will examine the safety and effectiveness of the Edwards SAPIEN 3/ SAPIEN 3 Ultra transcatheter heart valve (THV) versus careful observation (or clinical surveillance) in patients with severe aortic stenosis without symptoms.
The purpose of the trial is to compare results of patients that have their valves replaced early in the disease process versus patients that have the disease monitored.
Severe aortic stenosis with no symptoms
Aortic stenosis is caused by a buildup of calcium deposits on the valve leaflets, which causes the valve to narrow and reduce blood flow to the rest of your body.
Many patients with severe aortic stenosis experience symptoms, while others have no symptoms (or are asymptomatic). The symptoms of aortic stenosis are often misunderstood by patients as “normal” signs of aging. Many patients may appear to not have symptoms, but on closer examination, many do have symptoms.

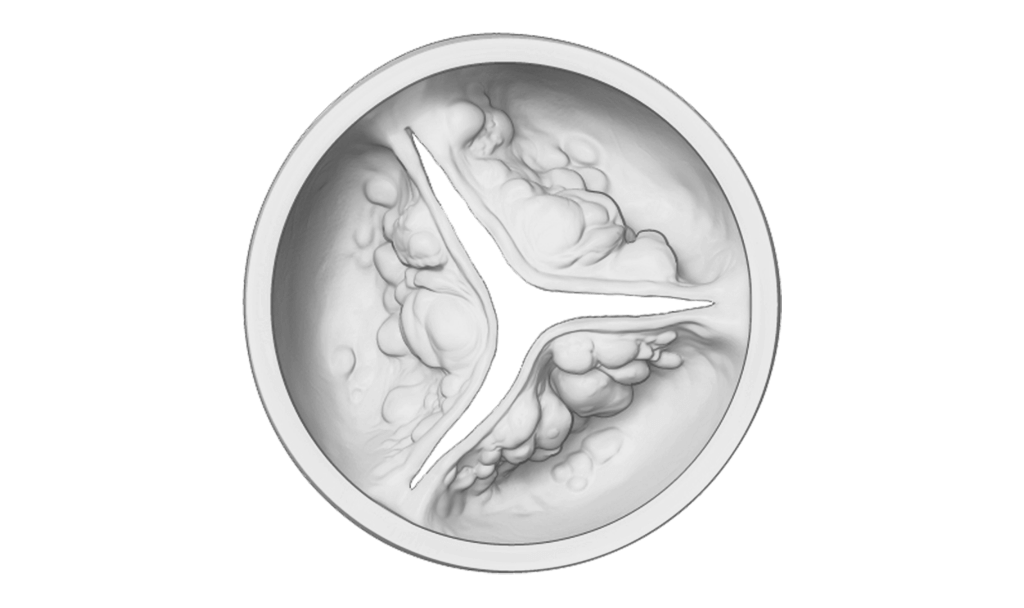
Diseased aortic valve (with calcium buildup)
Are you a candidate?

You may be eligible for this trial if:
- you are 65 years of age or older
- you have severe aortic stenosis
- you do not have symptoms
Your doctor or research coordinator will discuss with you the full list of criteria for participating in the trial.
For Patients who have severe aortic stenosis and have no symptoms, a treadmill stress test helps to understand if there are symptoms during mild exercise. This test involves walking on a treadmill while your heart, blood pressure, and breathing are monitored by your doctor.

Questions to ask your doctor

- What screening tests will be done?
- What are the possible risks and benefits of the TAVR procedure?
- What are the possible risks and benefits of monitoring my disease (or clinical surveillance)?
- How often will I need to have follow-up visits?
- How long will the trial last?


We're here for you
We are committed to providing the highest levels of customer service to help our patients improve their quality of life. For any questions, please contact the Edwards Patient Support Center. For details about the trial, visit NCT03042104 at ClinicalTrials.gov.
Give us a call
Send us an email
Caution: Investigational device
The Edwards SAPIEN 3 / Edwards SAPIEN 3 Ultra transcatheter heart valve is an investigational device when used in asymptomatic patients. Limited by Federal (USA) law to investigational use only. These devices are not available for marketing or commercial sale in the United States for asymptomatic patients.
