The ALT-FLOW II trial for heart failure


About the trial
The ALT-FLOW II clinical trial is studying the safety, performance, and effectiveness of the Edwards APTURE transcatheter shunt, a device designed to relieve symptoms due to certain types of heart failure. This trial is enrolling participants that are experiencing heart failure symptoms despite being on guideline-directed medical therapy.
Talk to your heart doctor and/or contact the Edwards Patient Support Center to learn more.

ALT-FLOW II Trial explained: a video for patients and caregivers
Types of heart failure
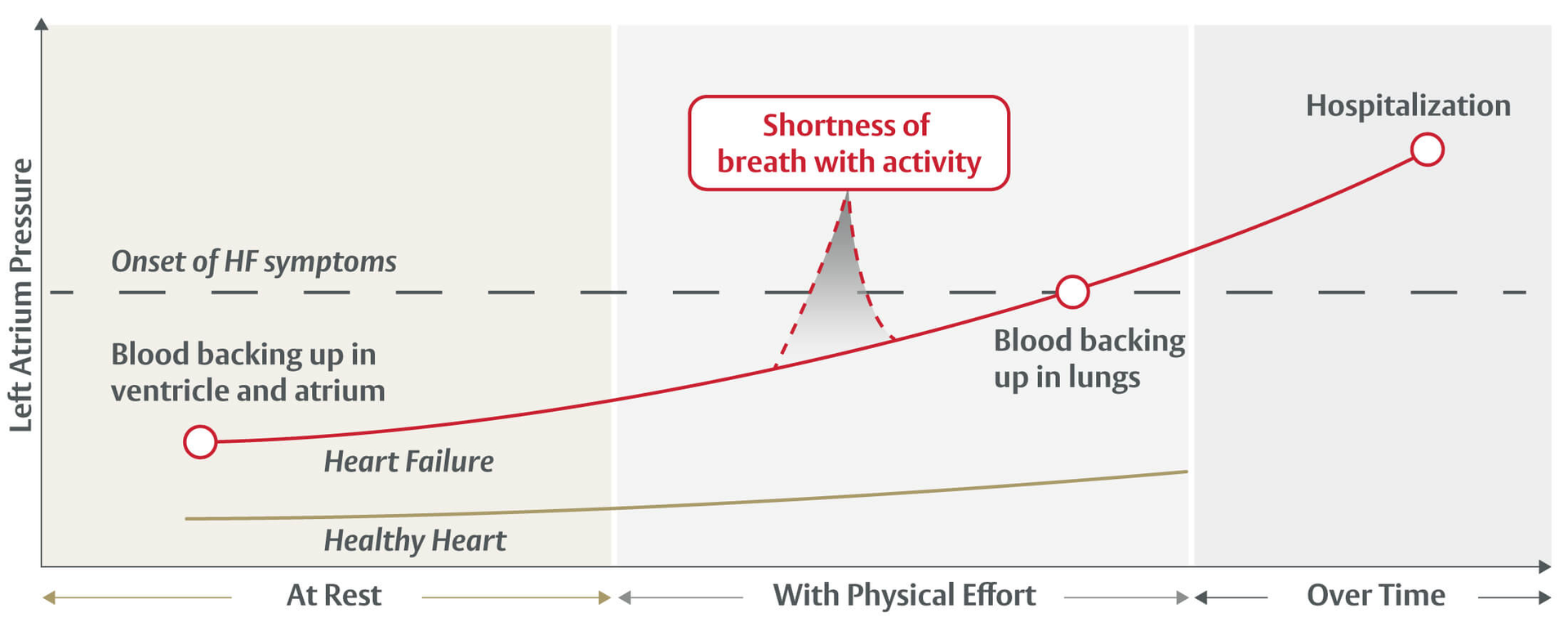
Heart failure is a chronic condition that occurs when the heart muscle is not working as well as it should. Heart failure can result from many different underlying causes. Despite the underlying cause people often experience similar signs and symptoms such as shortness of breath, fatigue, difficulty exercising, and swelling in the legs and ankles.
The ALT-FLOW II trial is enrolling participants who have heart failure that is due to their heart muscle's inability to relax and fill with blood normally. This can cause pressure within the heart, specifically within the left atrium, to rise.

Ejection fraction: what is it?
Left ventricular ejection fraction (LVEF) is often abbreviated to and discussed as ejection fraction (EF).
EF is a measure of how much blood the heart can pump out with each heart beat, and it is reported as a percentage (%). When diagnosing heart failure, the EF% is often used to characterize the type of heart failure so that it may be treated appropriately.
If someone is diagnosed with heart failure and their EF measurement is ≤ 40%, it means there is high likelihood that they have HFrEF. If their EF measurement is 41-49%, there is a high likelihood that they have HFmrEF. If their EF measurement is ≥ 50%, there is a high likelihood they have HFpEF. Individuals diagnosed with HFmrEF and HFpEF are being studied in the ALT-FLOW II trial.
What is the Edwards APTURE transcatheter shunt?
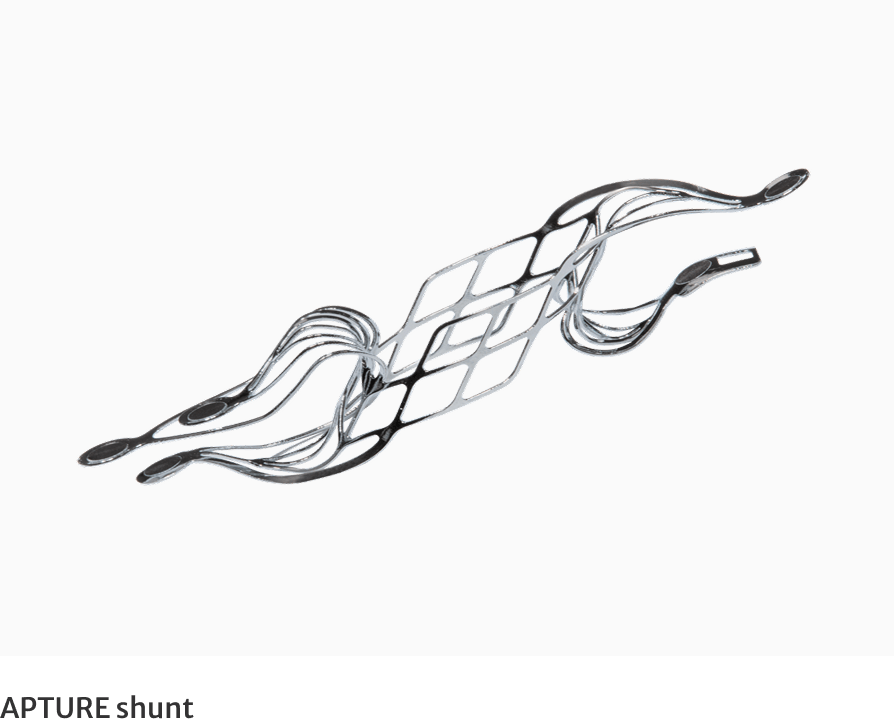
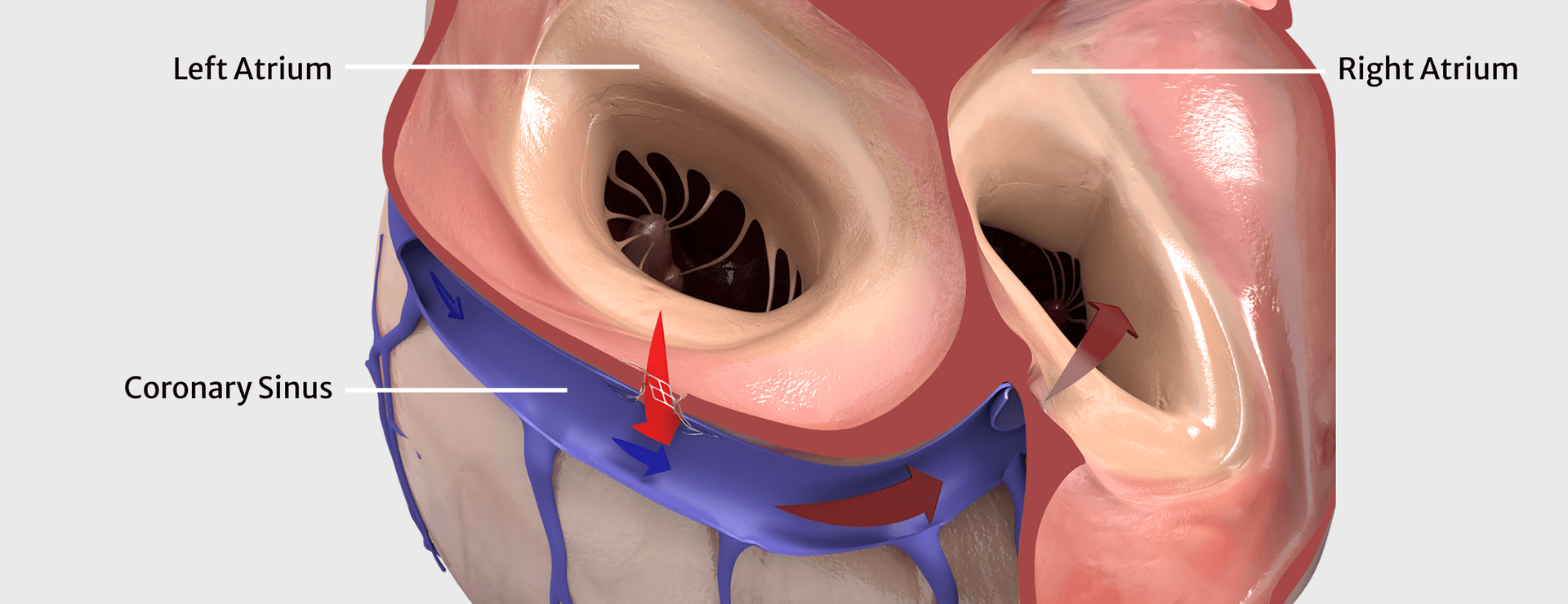
The investigational device being studied in this trial is the APTURE shunt, a metal device that is placed in the heart to create a channel allowing a small amount of blood to flow from a heart chamber with elevated pressure - the left atrium, to a heart chamber with lower pressure - the right atrium, via a blood vessel - the coronary sinus. This shunting of blood is intended to relieve the elevated pressure which can cause symptoms associated with HFmrEF and HFpEF.

Frequently Asked Questions: ALT-FLOW II clinical trial
Find a Trial Center Near You

Learn more: are you a trial candidate?
Find out more about this trial, NCT05686317, by visiting ClinicalTrials.gov.
Additionally you can contact the Edwards Patient Support Center.
Give us a call
Send us an email
CAUTION: Investigational device. Limited by Federal (or United States) law to investigational use.
Edwards Lifesciences is the sponsor of the ALT-FLOW II trial, NCT05686317
Edwards, Edwards Lifesciences, the stylized E logo, and APTURE are trademarks of Edwards Lifesciences Corporation or its affiliates. All other trademarks are the property of their respective owners.
