
About the trial

The JOURNEY trial will assess the safety and efficacy of the J-Valve transfemoral (TF) system in patients with symptomatic, severe (grade 3 or 4), native AR and AR-dominant mixed aortic valve disease, who are judged by a multidisciplinary heart team to be at high risk for open SAVR.
Contact our Patient Support Center to find a participating hospital near you
Aortic regurgitation

Aortic regurgitation (AR) occurs in approximately 0.4% of all adults, in 1% of individuals aged 65-74 years and 2% of individuals over 70 years of age.1
Symptomatic patients with chronic severe AR have a poor prognosis and should therefore undergo surgical aortic valve replacement (SAVR) per current guidelines. The guideline directed indications for patients with Grade 3 or 4 AR include these symptomatic patients as well as asymptomatic patients with evidence of left ventricular remodeling, including dysfunction.2,3

Trial design

*Multimodality diagnostic assessment to determine AR severity by echocardiography and cardiac magnetic resonance (CMR) criteria for indeterminate AR
Eligibility criteria
- Symptomatic according to New York Heart Association (NYHA) functional class (FC) II or higher
- Severe AR, defined as follows (Either A or B):
- A. Severe AR by Echocardiography (grade 3 or 4)
- B. Indeterminate AR, in addition to ANY ONE of the following:
- Cardiac magnetic resonance imaging (CMR)-derived aortic regurgitant fraction (RF) ≥43%
- CMR-derived RF ≥33% + left ventricular dilation (left ventricular end diastolic volume index (LVEDVi) >105 mL/m2 for men or LVEDVi > 96 mL/m2 for women)
- CMR-derived RF ≥33% + LV ejection fraction (LVEF) ≤55% or left-ventricular end-systolic volume index (LVESVi) ≥43 mL/m²
- Patient is judged by a multidisciplinary heart team to be at high risk for surgery
- Patient has suitable anatomy to accommodate the insertion and delivery of the J-Valve TF System
Study device
Edwards J-Valve TAVR-AR System
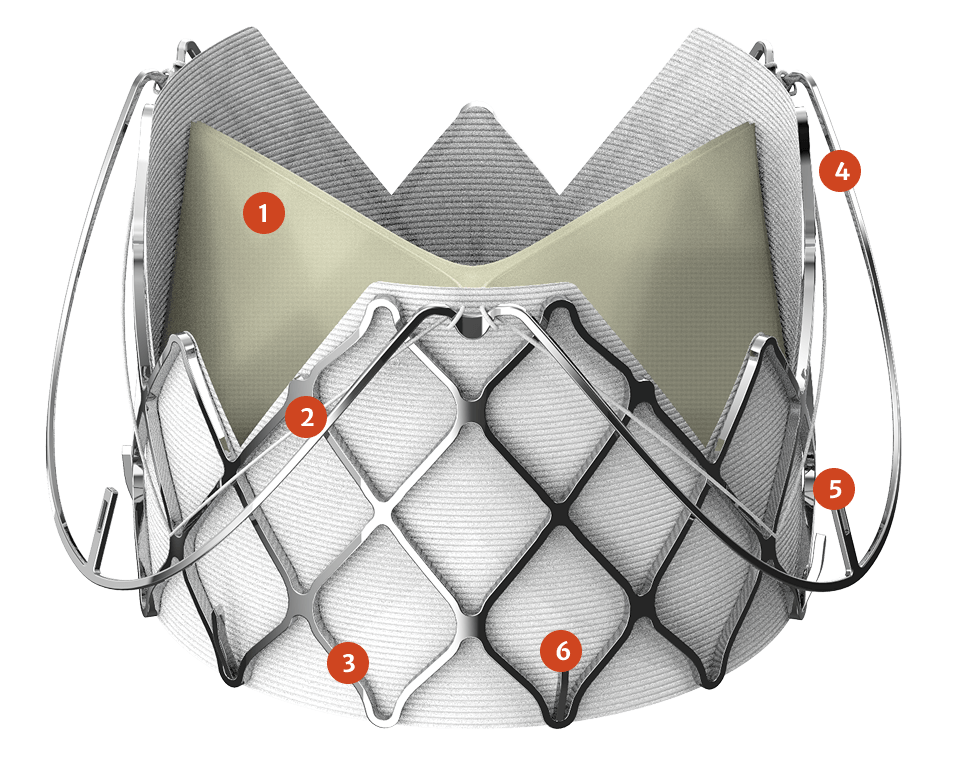
- 1Bovine pericardium leaflets
- 2Interconnecting suture
- 3Nitinol frame
- 4Anchor ring
- 5Latch (attachment to delivery device (x3))
- 6Grip feature (x6)

The J-Valve TAVR-AR system procedural animation

We're here for you
We are committed to providing the highest levels of customer service to help our patients improve their quality of life. For any questions, please contact the Edwards Patient Support Center.
For details about the trial, visit NCT06455787 at Clinical Trials.gov.
Give us a call
Send us an email

