Magna Mitral Ease valve

Carpentier-Edwards PERIMOUNT Magna Mitral Ease valve
Built upon the unique and proven PERIMOUNT valve design, the Magna Mitral Ease valve gives you and your patients:
- Ultra-low profile, with reduced ventricular projection by up to 40%‡
- Exceptional long-term durability1-8
- Ease of implant
The Magna Mitral Ease valve is built upon the proven, time-tested PERIMOUNT valve design, with unique design elements including:
- Mathematically modeled, bioengineered design. Intended to optimize implantability, hemodynamics and long-term durability
- Flexible cobalt-chromium alloy stent. Absorbs energy to reduce leaflet stress
- Three independent bovine pericardial leaflets. Matched for thickness and elasticity to optimize stress distribution
‡ As compared to the Carpentier-Edwards PERIMOUNT Theon mitral valve.
Magna Mitral Ease heart valve
| Model 7300TFX Nominal Specifications (mm) | Size 25 | Size 27 | Size 29 | Size 31 | Size 33 |
| 1. Inflow Orifice Diameter (mm) | 23.0 | 25.0 | 27.0 | 29.0 | 29.0 |
| 2. Effective Orifice Diameter (mm) | 18.0 | 20.0 | 22.0 | 22.5 | 22.5 |
| 3. Stent Diameter (Wireform, mm) | 25 | 27 | 29 | 31 | 31 |
| 4. External Stent Post Diameter (Tip, mm) | 29 | 31 | 34 | 35 | 35 |
| 5. Valve Housing External Diameter (mm) | 27.5 | 29.5 | 31.5 | 33.5 | 33.5 |
| 6. External Sewing Ring Diameter (mm) | 36.0 | 37.5 | 40.0 | 42.0 | 44.5 |
| 7. Effective Profile Posterior (mm) | 10 | 10.5 | 11 | 11.5 | 11.5 |
| 8. Effective Profile Anterior (mm) | 7 | 7.5 | 8 | 8.5 | 8.5 |
| 9. Total Profile Height (mm) | 15 | 16 | 17 | 18 | 18 |

References
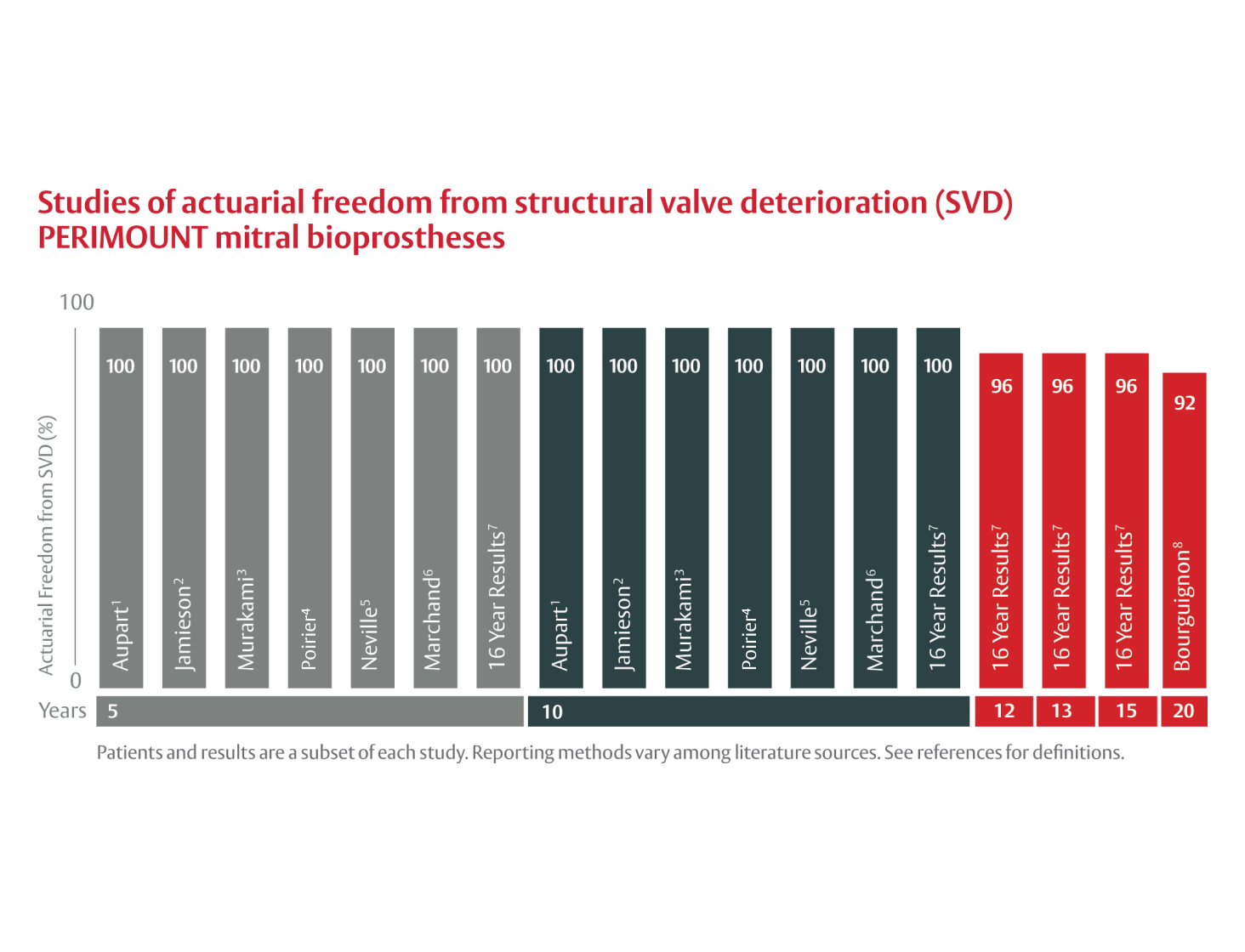
- Aupart MR, Neville PH, Hammami S, et al. Carpentier-Edwards pericardial valves in the mitral position: ten-year follow-up. J Thorac Cardiovasc Surg. 1997;113(3):492-498. doi: 10.1016/S0022-5223(97)70361-1 (Freedom from structural deterioration; n = 150; mean age = 62.9 ± 11.9 yrs)
- Jamieson WRE, Marchand MA, Pelletier CL, et al. Structural valve deterioration in mitral replacement surgery: comparison of Carpentier-Edwards supra-annular porcine and perimount pericardial bioprostheses. J Thorac Cardiovasc Surg. 1999;118(2):297-304. doi: 10.1016/S0022-5223(99)70220-5 (Freedom from explant due to structural valve deterioration; n = 429; mean age = 60.7 ± 11.7 yrs)
- Murakami T, Eishi K, Nakano S, et al. Aortic and mitral valve replacement with the Carpentier-Edwards pericardial bioprosthesis: 10-year results. J Heart Valve Dis. 1996;5(1):45-49. (Freedom from structural deterioration; n = 57; mean age = 55.1 ± 13.2 yrs)
- Poirer NC, Pelletier LC, Pellerin M, et al. 15-year experience with the Carpentier-Edwards pericardial bioprosthesis. Ann Thorac Surg. 1998;66(6 Suppl):S57-S61. doi:10.1016/s0003-4975(98)01110-2 (Freedom from structural deterioration; n = 214; mean age = 65 ± 23 yrs)
- Neville PH, Aupart MR, Diemont FF, et al. Carpentier-Edwards pericardial bioprosthesis in aortic or mitral position: a 12-year experience. Ann Thorac Surg. 1998;66(6 Suppl):S143-S147. doi: 10.1016/s0003-4975(98)01122-9 (Freedom from structural deterioration; n = 182; mean age = 63.9 ± 11.5 yrs)
- Marchand MA, Aupart MR, Norton R, et al. Fifteen-year experience with the mitral Carpentier-Edwards PERIMOUNT pericardial bioprosthesis. Ann Thorac Surg. 2001;71(5 Suppl):S236-S239. doi: 10.1016/s0003-4975(01)02550-4 (Freedom from structural valve deterioration; n = 435; mean age = 60.7 ± 11.6 yrs)
- Bourguignon T, Bouquiaux-Stablo AL, Loardi C, et al. Very late outcomes for mitral valve replacement with the Carpentier-Edwards pericardial bioprosthesis: 25-year follow-up of 450 implantations. J Thorac Cardiovasc Surg. 2014;148(5):2004-2011.e1. doi: 10.1016/j.jtcvs.2014.02.050 (Freedom from explant due to structural valve deterioration; n = 404; mean age = 68.0 ± 10.4 yrs)
- Carpentier-Edwards PERIMOUNT pericardial bioprosthesis 16-year results. Data on file at Edwards Lifesciences, 2003. (Freedom from explant due to structural valve deterioration; n = 435; mean age = 60.7 ± 11.6 yrs)
Important Safety Information
Carpentier-Edwards PERIMOUNT Mitral Bioprostheses
Indications: For use in patients whose mitral valvular disease warrants replacement of their natural or previously placed prosthetic mitral valve.
Contraindications: Do not use if surgeon believes such would be contrary to the patient’s best interests.
Complications and Side Effects: Stenosis, regurgitation, endocarditis, hemolysis, thromboembolism, valve thrombosis, nonstructural dysfunction, structural valve deterioration, anemia, arrhythmia, hemorrhage, transient ischemic attack/ stroke, congestive heart failure, myocardial infarction, angina, ventricular perforation by stent posts any of which could lead to reoperation, explantation, permanent disability, and death.
CAUTION: USA law restricts this device to sale by or on the order of a physician. See instructions for use for full prescribing information.