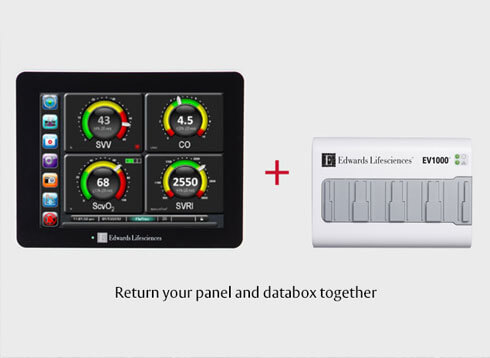
- Collect all Edwards equipment to be returned that is listed in the Returned Goods Authorization (RGA).
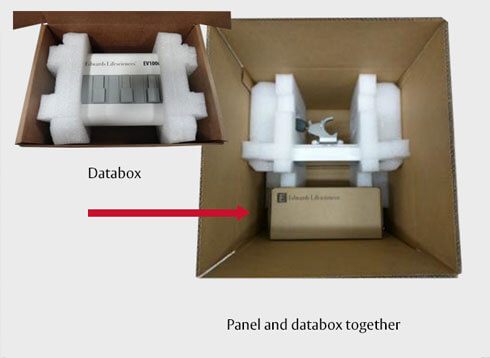
- Place your Edwards equipment into the shipping box with secure padding (or with the Edwards provided packaging, where applicable). See below for further packing instructions/diagrams for each product.
- Note: Do not return any component not listed on your RGA. Edwards will not replace or return any components that are not listed in the RGA.