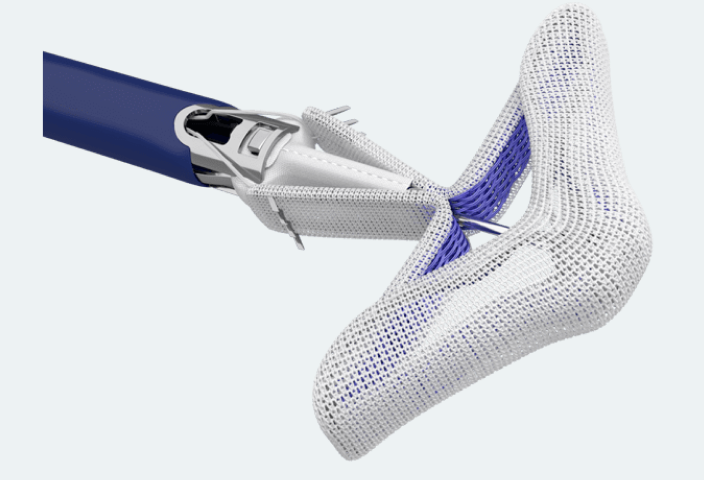
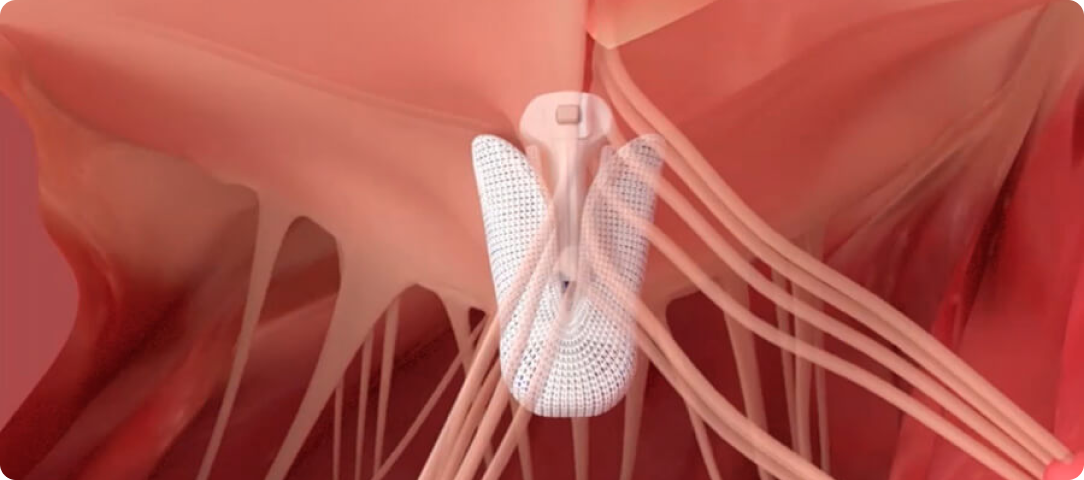
PASCAL Implant
Designed to minimize stress on leaflets and
reduce risk of leaflet tears
For treating mitral and tricuspid regurgitation
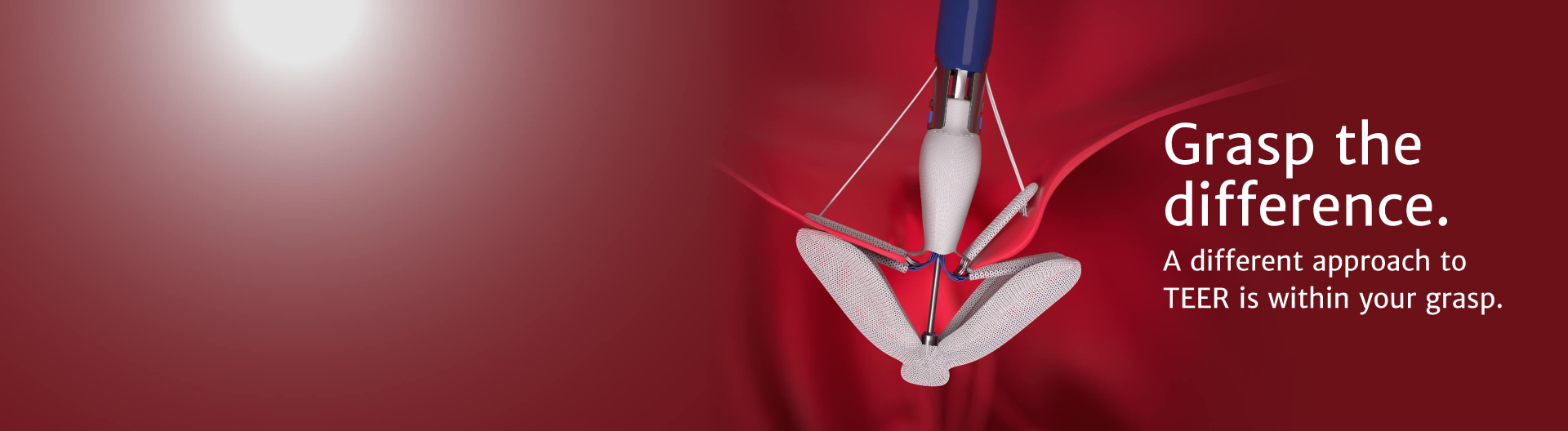
Introducing the latest advancement from Edwards Lifesciences in transcatheter mitral and tricuspid therapies
Designed to minimize stress on leaflets and
reduce risk of leaflet tears
Features a narrower design profile to help you optimize your
treatment of mitral and tricuspid regurgitation
Treat your patients with mitral and
tricuspid regurgitation with the latest
transcatheter innovation from Edwards
Lifesciences
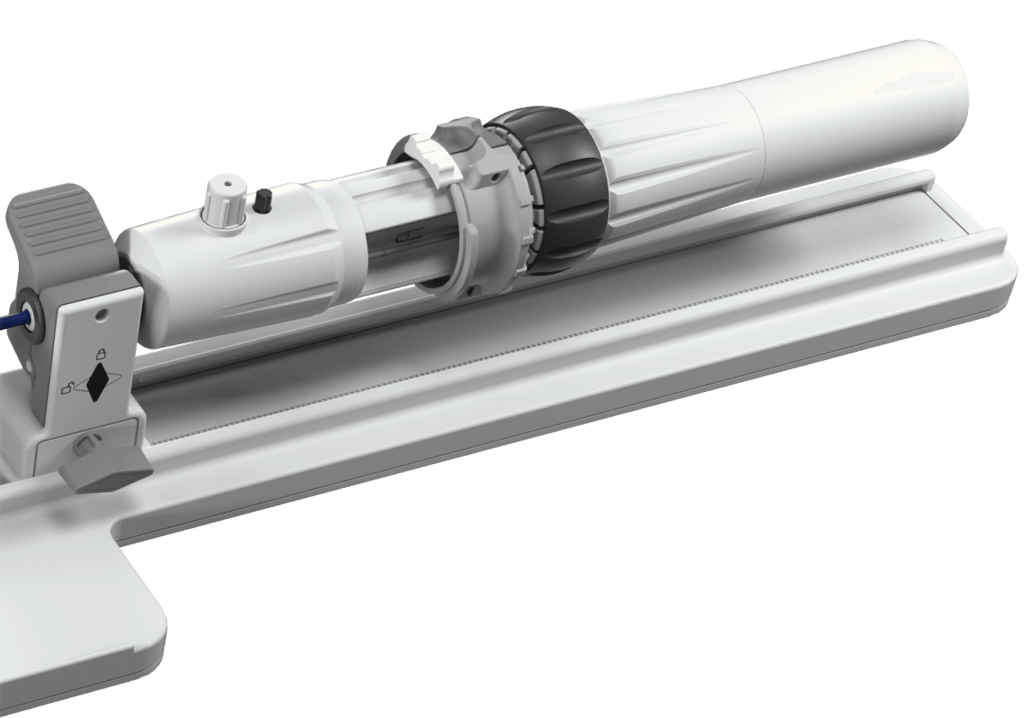
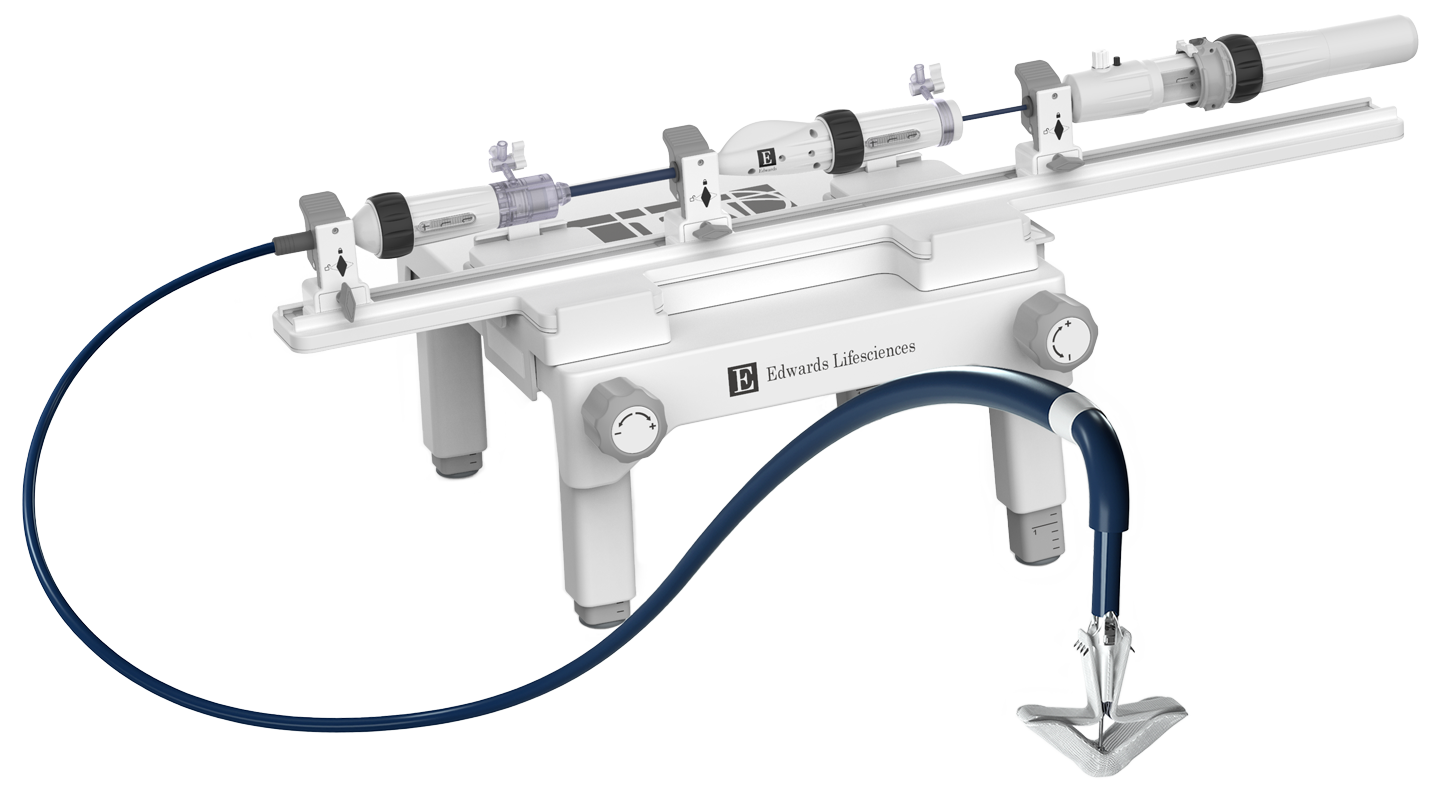
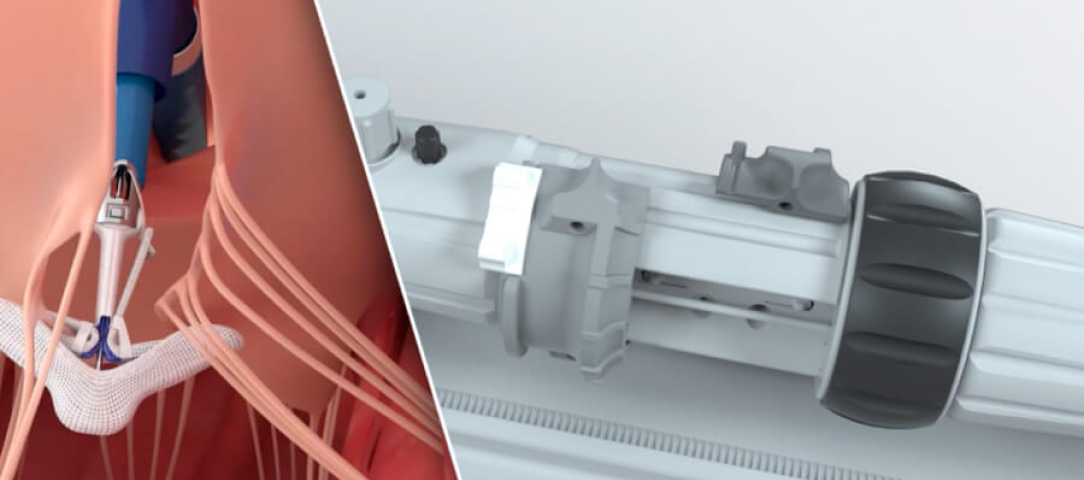
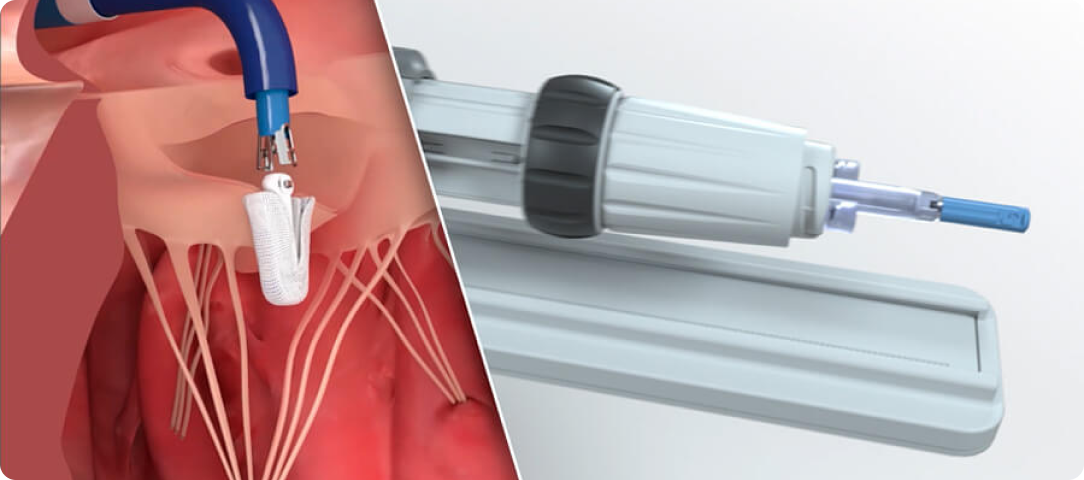
Advanced catheter and handle design facilitates smooth navigation and implant deployment¶
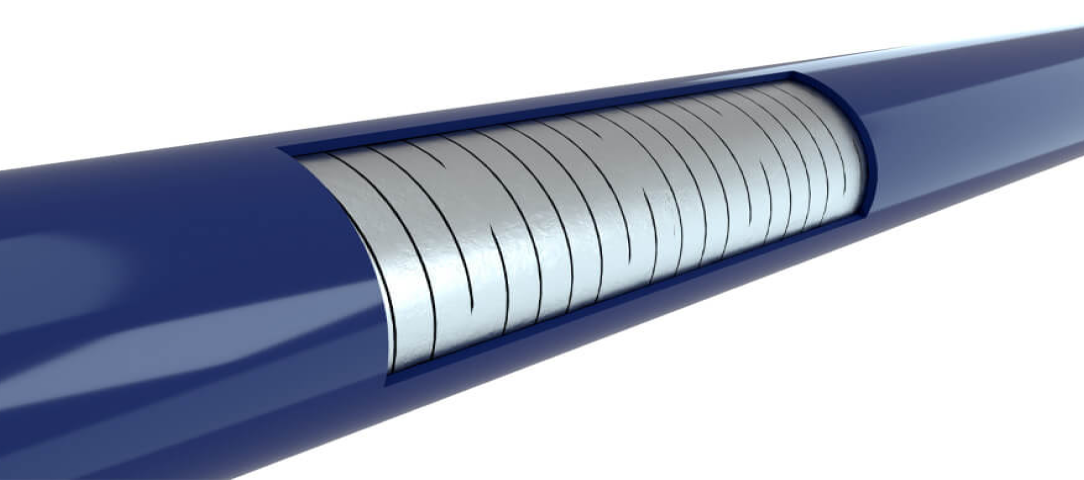
Responsive catheter design with optimized torque transfer to facilitate implant placement§

Handle controls engineered for intuitive manipulation, allowing the user to focus on treating the patient¶
*Performance data on file and marketing evaluation.
§Design and performance data on file and marketing evaluation.
¶Design data on file and marketing evaluation.

†Performance data on file.
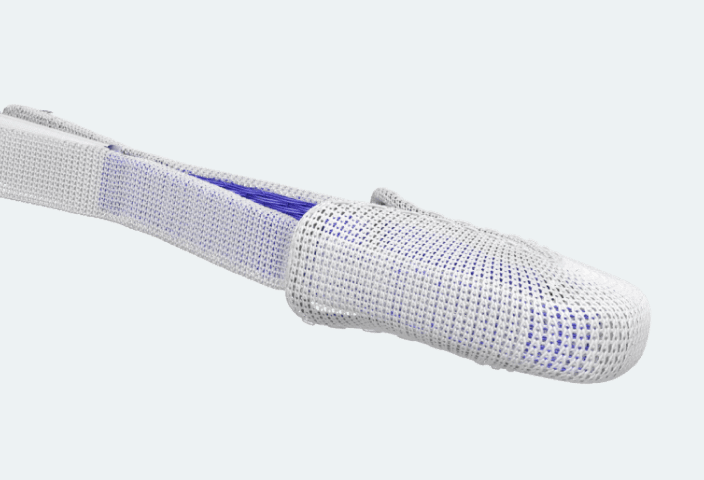
Enhance leaflet capture with atraumatic reclasp capabilities
Each paddle features a single row of retention elements to clasp, reclasp, and preserve leaflets

Close the implant to conform to native anatomy and flex during the cardiac cycle
‡Performance and simulation data on file.
†Performance data on file.
Learn about outcomes from a large randomized trial, early feasibility studies (EFS) and real-world studies
Currently published data from some studies were collected before the availability of the PASCAL Precision delivery system

Prospective, multicenter, randomized controlled trial to directly compare the safety and effectiveness of two contemporary transcatheter edge-to-edge repair (TEER) therapies







All-cause mortality

Heart failure hospitalization

New York Heart Association Functional Class

KCCQ Score
aTEE was used for baseline qualification of 5 patients.
Retrospective, observational, multicenter study including 524 patients




NYHA class
TR = Tricuspid Regurgitation
*Expert opinions, advice and all other information expressed represent contributors' views and not necessarily those of Edwards Lifesciences


For a listing of indications, contraindications, precautions, warnings, and potential adverse events, please refer to the Instructions for Use (consult eifu.edwards.com where applicable).