The Surge

Anticoagulation Considerations for Choosing a Tissue or Mechanical Heart Valve

Speaking with patients about their surgical valve options can require an overview of multiple quality of life variables, including the use of anticoagulation therapy for mechanical valves. This article briefly covers some of the anticoagulation considerations for choosing a tissue or mechanical heart valve.
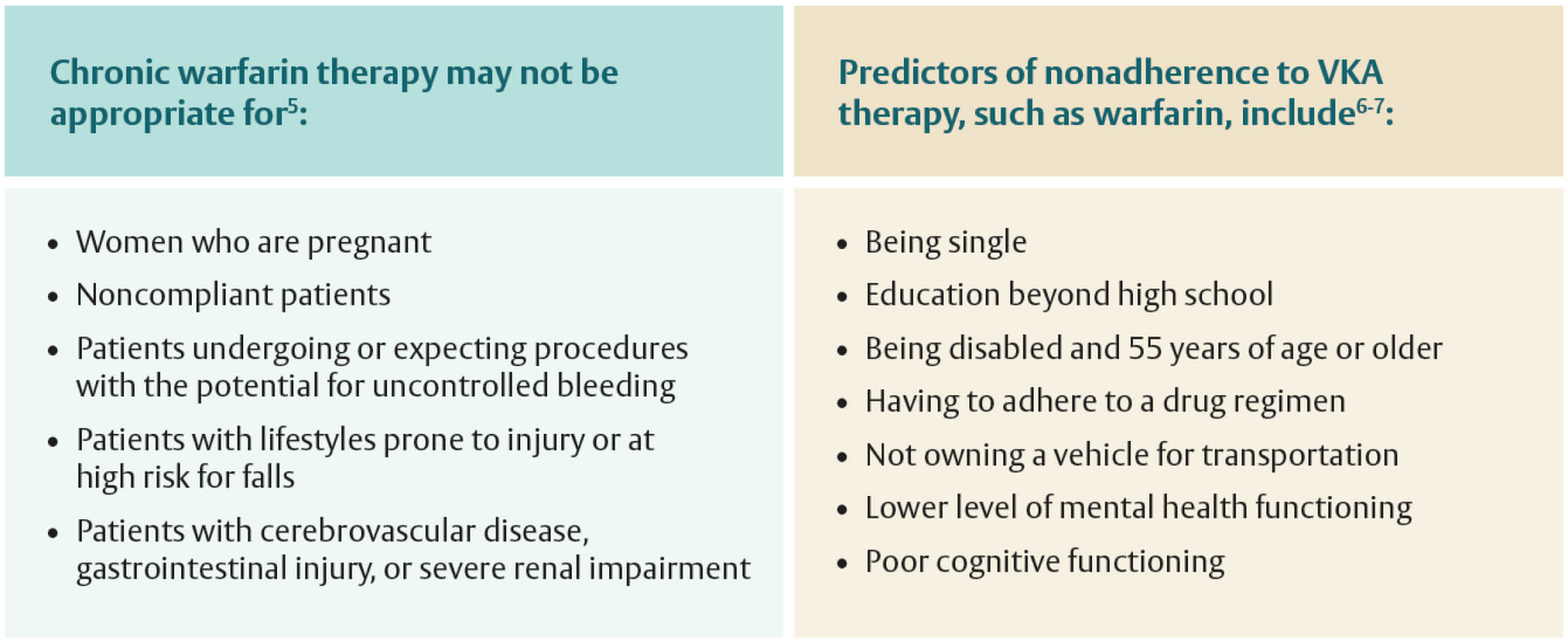
Vitamin K antagonists such as warfarin are the recommended anticoagulation therapy for patients who have mechanical valves1
- Non-vitamin K oral anticoagulants are not approved for patients who have mechanical valves1,2
- All major valvular heart disease (VHD) societal guidelines recommend lifelong therapy with vitamin K antagonists (VKAs), which, to be successful, require patient education and patient adherence to attain and maintain a therapeutic international normalized ratio (INR)1-4
- Vitamin K antagonists may not be appropriate for specific patient populations, as shown in the tables below
The challenges of lifetime anticoagulant therapy for mechanical valve patients may include1:
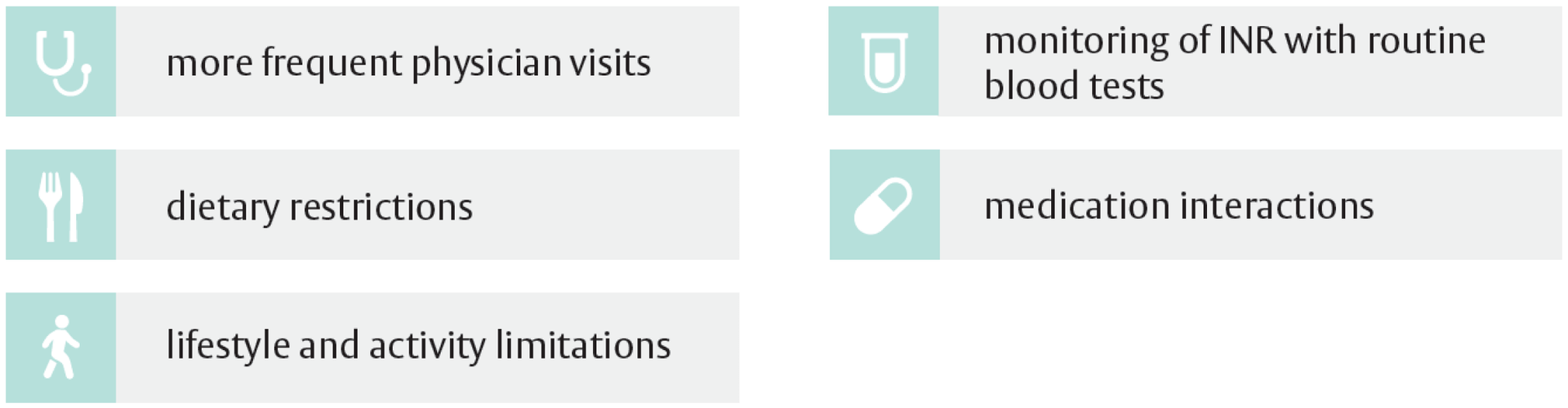
Difficulty in achieving and sustaining goal INR1,5
The 2020 ACC/AHA VHD guidelines recommend an INR goal of 3.0 (2.5-3.5) for patients who have a mitral mechanical valve and for those who have an aortic mechanical valve and higher thromboembolic risk. For current-generation mechanical valves in the aortic position, an INR of 2.5 (2.0-3.0) is recommended. Maintaining a stable INR, however, is challenging for patients on VKA therapy. When the INR value is above the therapeutic range, patients are at a higher risk of major bleeding events, and when the INR value is below the therapeutic range, patients are at a higher risk of thrombotic events, such as stroke.
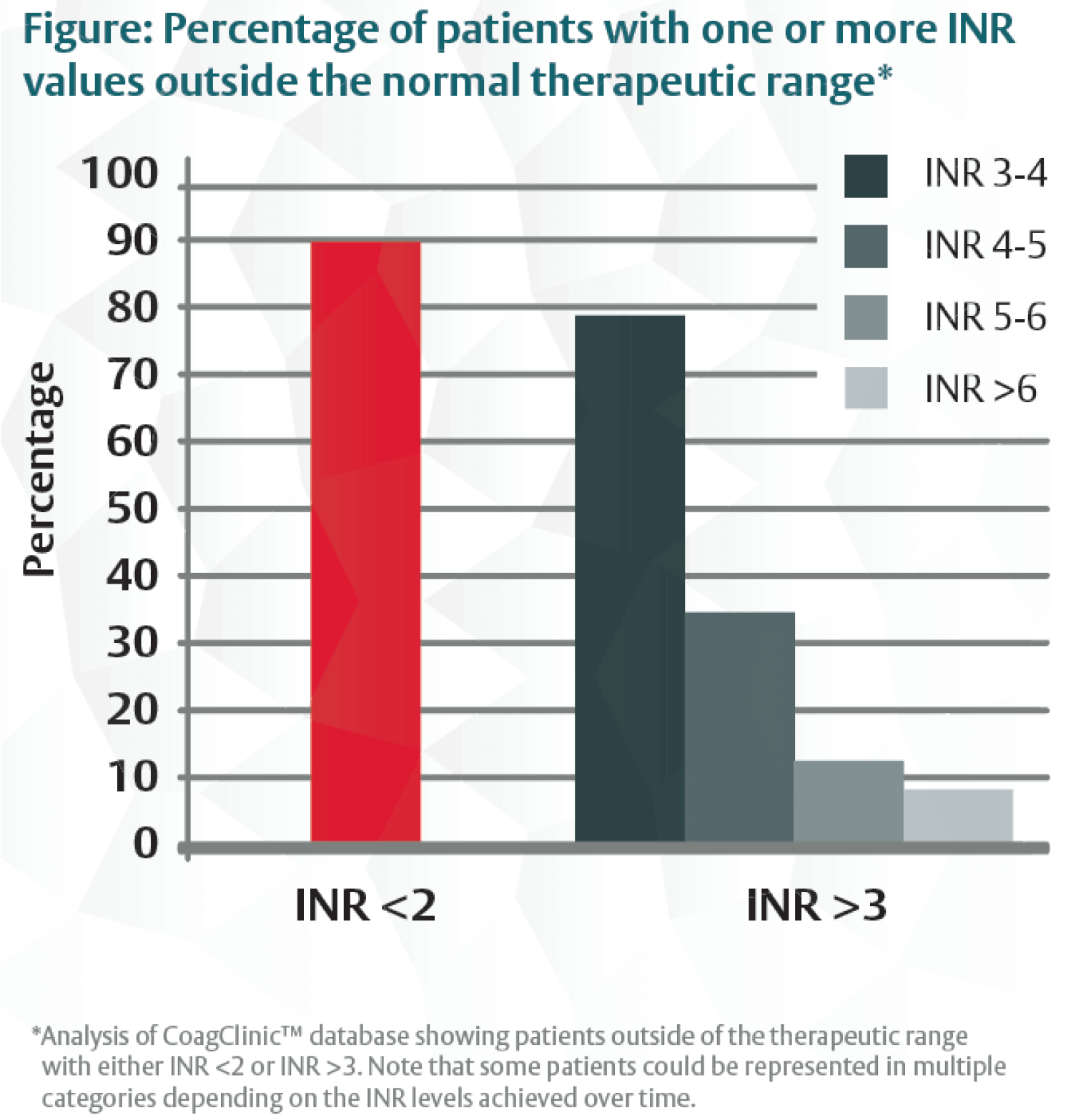
In a retrospective analysis of 9433 patients who had been using warfarin for more than 6 months (for a target INR of 2-3), 90% had at least one INR measurement lower than 2 and approximately 8% to 79% had at least one in the range of 3 to 6 or higher (Figure).
However, tissue valves do not require lifelong anticoagulation1,2,4
United States and EU VHD guidelines recommend implantation of a tissue valve in patients who have a desire to avoid anticoagulation therapy, regardless of age.1-2
In addition, all major VHD societal guidelines recommend tissue valves when good-quality anticoagulation is unlikely to be achieved, owing to factors such as patient adherence, availability, previous major bleeding, comorbidities, unwillingness, lifestyle, and occupation.1,2,4
Related Articles
References
- Otto CM, Nishimura RA, Bonow RO, et al. J Am Coll Cardiol. 2021;77(4):e25-e197.
- Vahanian A, Beyersdorf F, Praz F, et al. Eur J Cardiothorac Surg. 2021;60:727-800.
- Schein JR, White CM, Nelson WW, et al. Thromb J. 2016;14(1):14.
- Izumi C, Eishi K, Ashihara K, et al. Circ J. 2020;84(11):2037-2119.
- Bristol Myers Squibb. U.S. Food and Drug Administration website. Accessed November 4, 2021.
- Orensky IA, Holdford DA. Pharmacotherapy. 2005;25:1801-1808.
- Platt AB, Localio AR, Brensinger CM, et al. Pharmacoepidemiol Drug Saf. 2008;17(9):853-860.
Important Safety Information
Carpentier-Edwards PERIMOUNT Aortic/Mitral Bioprostheses
Indications: For use in patients whose aortic or mitral valvular disease warrants replacement of their natural or previously placed prosthetic valve.
Contraindications: Do not use if surgeon believes such would be contrary to the patient’s best interests.
Complications and Side Effects: Stenosis, regurgitation, endocarditis, hemolysis, thromboembolism, valve thrombosis, nonstructural dysfunction, structural valve deterioration, anemia, arrhythmia, hemorrhage, transient ischemic attack/ stroke, congestive heart failure, myocardial infarction, angina, ventricular perforation by stent posts (mitral valve only), any of which could lead to reoperation, explantation, permanent disability, and death.
CAUTION: Federal (USA) law restricts these devices to sale by or on the order of a physician. See instructions for use for full prescribing information.
Edwards, Edwards Lifesciences, the stylized E logo, Carpentier-Edwards, Carpentier-Edwards PERIMOUNT, PERI, and PERIMOUNT are trademarks of Edwards Lifesciences Corporation or its affiliates. All other trademarks are the property of their respective owners.
© 2022 Edwards Lifesciences Corporation. All rights reserved. PP--US-7696 v1.0
Edwards Lifesciences • One Edwards Way, Irvine CA 92614 USA • edwards.com


