IntraClude device

IntraClude device
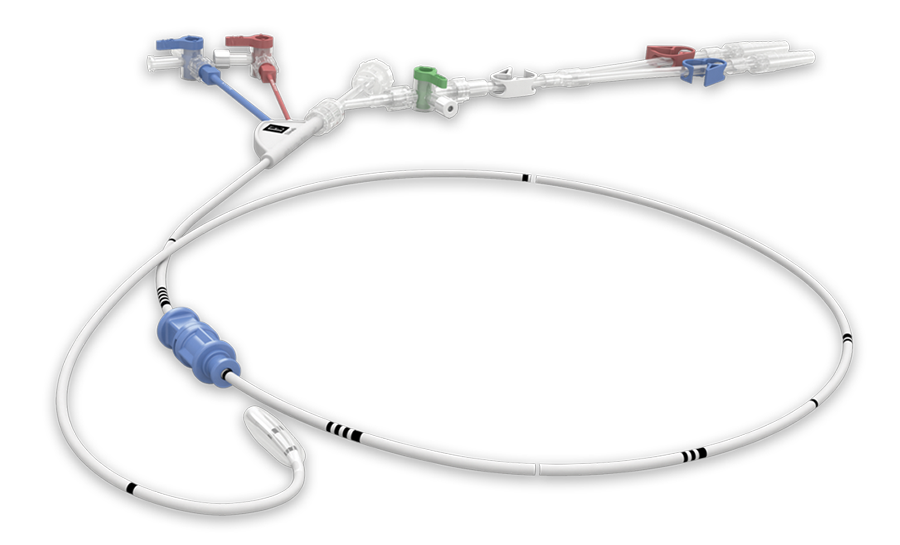
The IntraClude intra-aortic occlusion device is indicated for use in patients undergoing cardiopulmonary bypass. The IntraClude intra-aortic occlusion device occludes and vents the ascending aorta when the balloon is inflated. The device’s central lumen allows delivery of cardioplegia to arrest the heart. The pressure lumen allows monitoring of the aortic root pressure.
- 10.5 Fr (3.5 mm), triple-lumen, 100 cm long catheter
- Designed to occlude the ascending aorta in order to partition the aortic root from arterial circulation
- Balloon expands to occlude a range of aorta sizes from 20 to 40 mm
- Designed to be used in the femoral approach with:
- 21 Fr Edwards EndoReturn arterial cannula (ER21B)
- 23 Fr Edwards EndoReturn arterial cannula (ER23B)
- 19 Fr Edwards introducer sheath (IS19A)
- The shaft is provided with an extended strain relief designed to prevent kinking
Contents:
- 1 IntraClude intra-aortic occlusion device
- 1 Syringe (35 ml)
- 1 Guidewire (200 cm)
- 1 Blue stripe pressure tubing
- 1 Red stripe pressure tubing
1 / 4
Models & specifications
| Model | Description | Size | Image |
| ICF100 | IntraClude intra-aortic occlusion device | 10.5 Fr |  |
Medical device for professional use
Medical device for professional use
For a listing of indications, contraindications, precautions, warnings, and potential adverse events, please refer to the Instructions for Use (consult eifu.edwards.com where applicable).




