
people in Canada are estimated to have clinically relevant TR5,6
Feb 22 is Heart Valve Disease Awareness Day — Learn more about the HVD symptoms at Listen to your Heart

Medications may treat symptoms but not the TR itself, which can continue to progress. Reducing TR severity may improve patient quality of life.2,4

people in Canada are estimated to have clinically relevant TR5,6

of patients with mild or trivial TR progressed to moderate or severe TR in ~3 years§,7

estimated mortality in patients with severe TR within 1 year of diagnosis8, 9
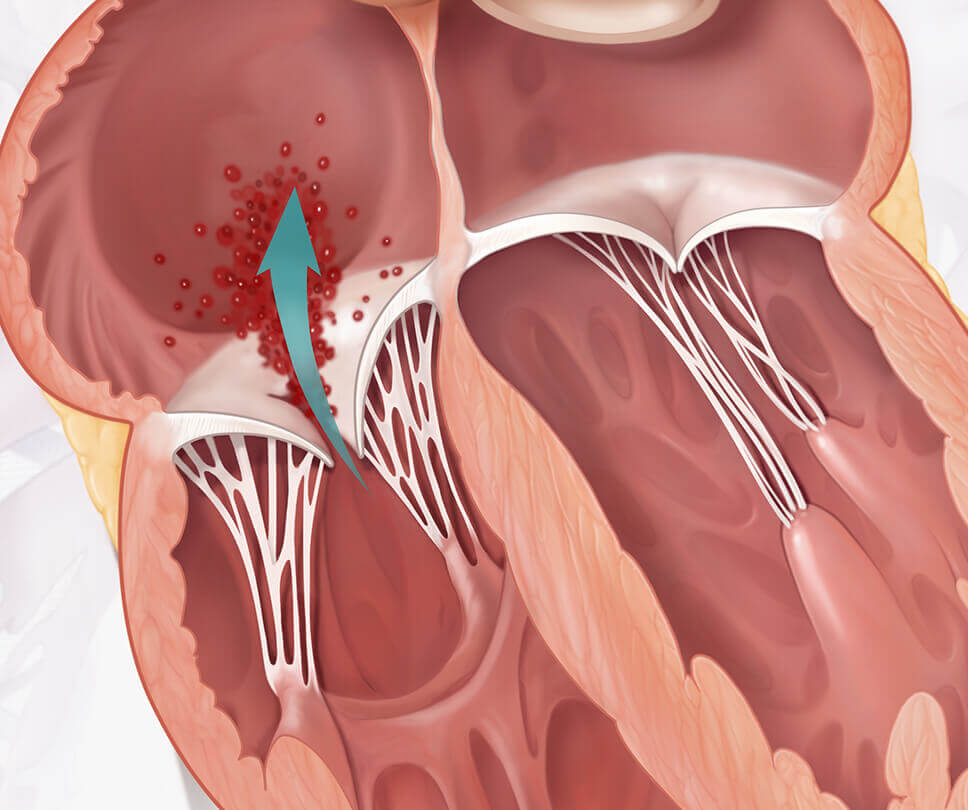
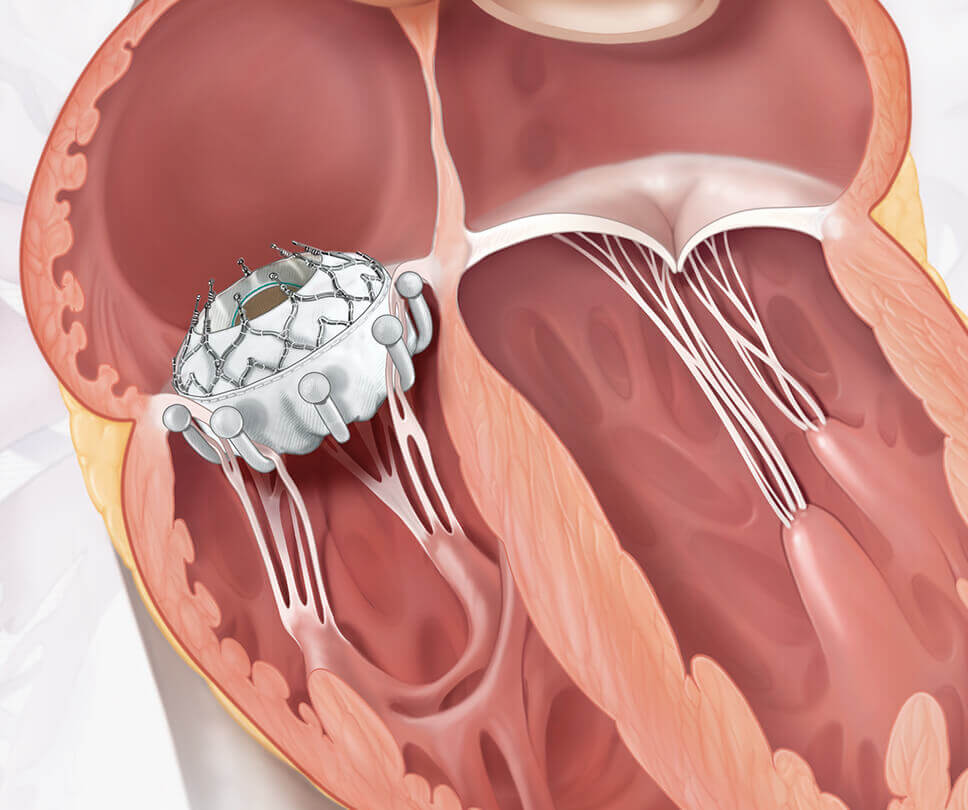
Move the arrow left or right to see the comparison between a heart with tricuspid regurgitation and one with the EVOQUE valve


Reducing TR severity may improve patient quality of life2,4
§Based on a retrospective echocardiographic analysis of Israeli patients (n=1,552).
The EVOQUE system is the world's first transcatheter tricuspid valve replacement therapy
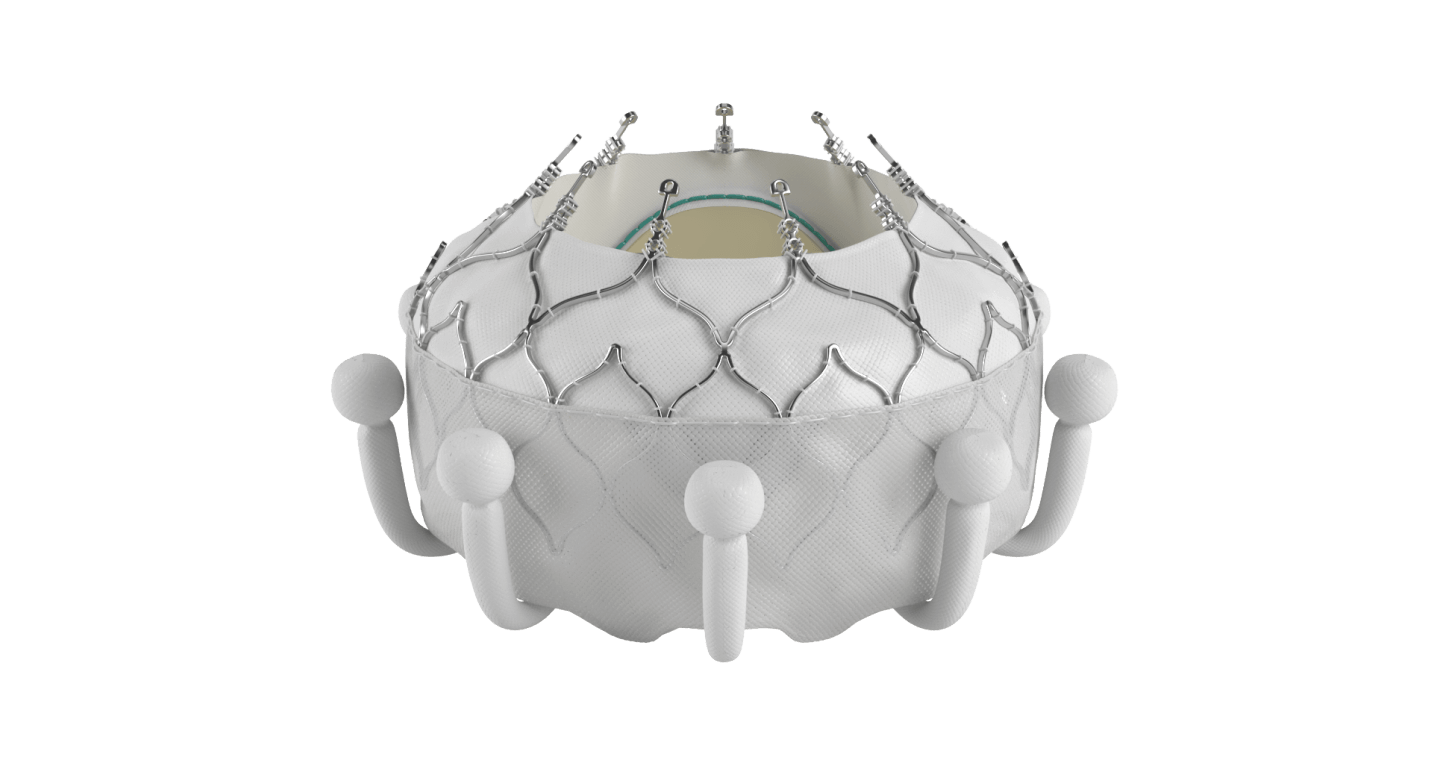
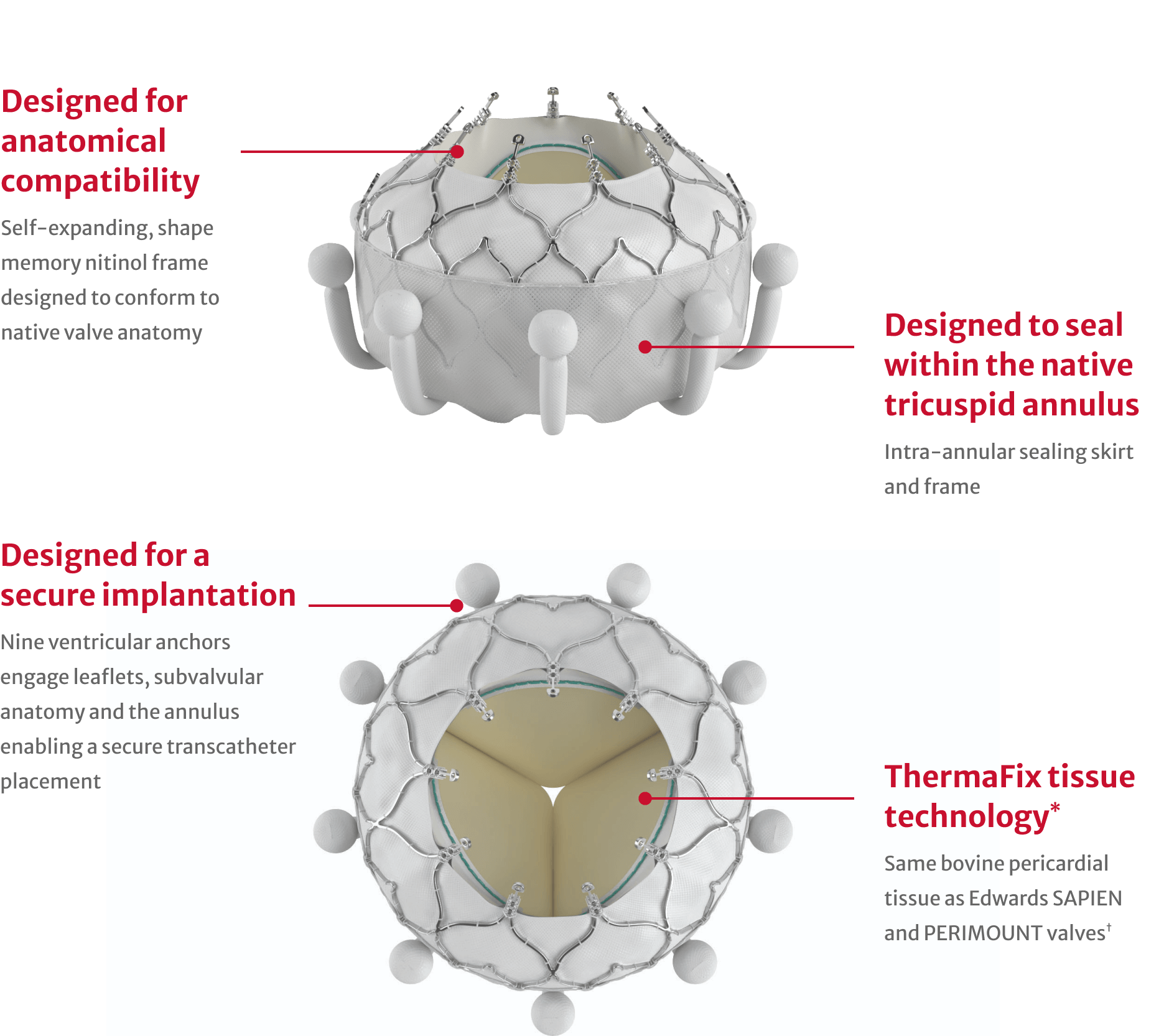
*No clinical data are available that evaluates long-term impact of the Carpentier-Edwards ThermaFix tissue process in patients.
†Excluding Edwards SAPIEN 3 Ultra RESILIA valves.

Procedural efficiency with an average device time of approximately one hour
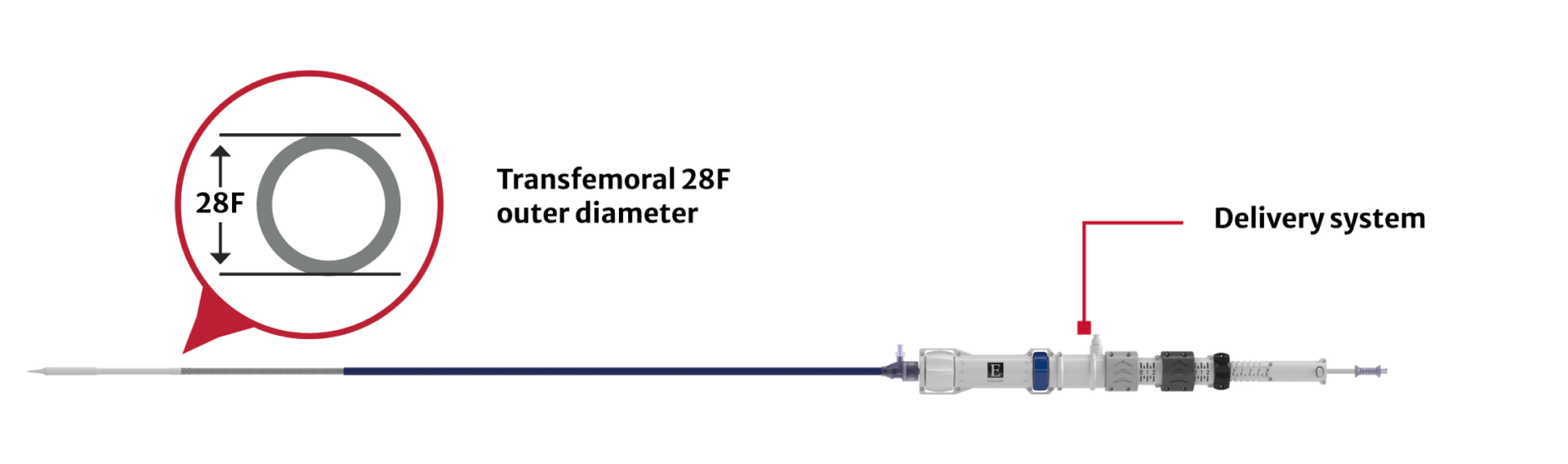
Same delivery system for all valve sizes
Superior clinical benefit and consistent TR resolution for patients with EVOQUE TTVR

The TRISCEND II trial is a prospective multi-center randomized controlled trial evaluating the safety and effectiveness of transcatheter tricuspid valve replacement with the EVOQUE system plus optimal medical therapy compared to optimal medical therapy alone in patients with symptomatic severe TR. These data pertain to the entire cohort of 400 patients at 1 year.
The TRISCEND II trial successfully met the composite primary safety and effectiveness endpoint at 1 year.
Greater likelihood of clinical benefit with EVOQUE TTVR vs medical therapy alone
(95% CI, 1.56 to 2.62; p<0.001)

Greater likelihood of clinical benefit with EVOQUE TTVR vs medical therapy alone
(95% CI, 1.56 to 2.62; p<0.001)


Composite Endpoint

Win Ratio Analysis
Greater likelihood of clinical benefit with EVOQUE TTVR vs medical therapy alone
(95% CI, 1.56 to 2.62; p<0.001)
Greater likelihood of clinical benefit with EVOQUE TTVR vs medical therapy alone
(95% CI, 1.56 to 2.62; p<0.001)
≤mild TR with EVOQUE TTVR at 1 year
median device time




TR Reduction

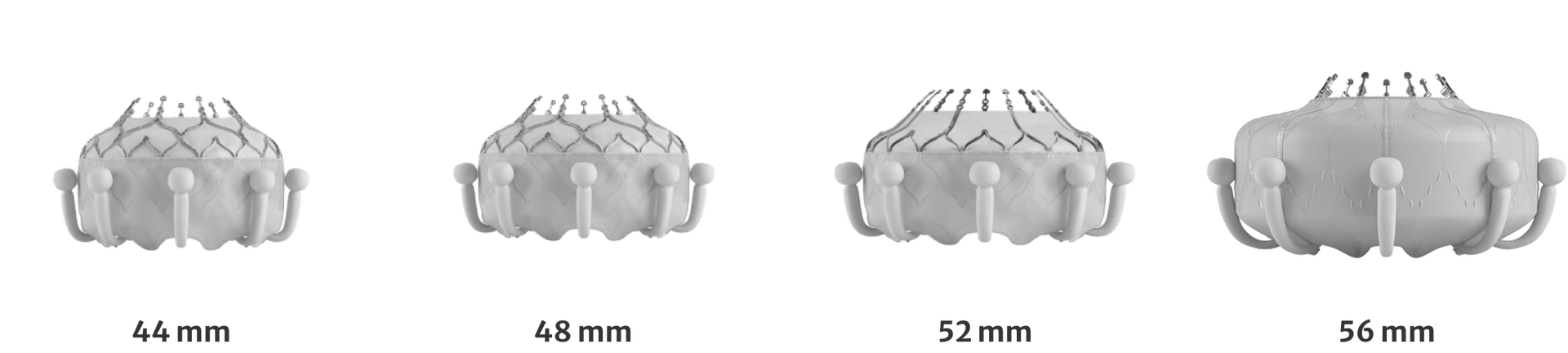
Treats a diverse patient population

Treats a broad patient population
≤mild TR with EVOQUE TTVR at 1 year
median device time
Favorable trends observed in all-cause mortality and heart failure hospitalizations at 1 year between EVOQUE TTVR and medical therapy alone.
with EVOQUE TTVR vs 15.2% with medical therapy alone

with EVOQUE TTVR vs 26.1% with medical therapy alone

Cardiovascular mortality
Stroke
Severe bleeding
New pacemaker implant in pacemaker-naïve patients


Kaplan-Meier All-Cause Mortality

Kaplan-Meier Heart Failure Hospitalization

30-day Safety profile
with EVOQUE TTVR vs 15.2% with medical therapy alone
with EVOQUE TTVR vs 26.1% with medical therapy alone
Cardiovascular mortality
Stroke
Severe bleeding
New pacemaker implant in pacemaker-naïve patients
At 1-year follow-up compared to baseline, patients treated with EVOQUE TTVR experienced meaningful and sustained TR improvements in quality-of-life, functional status, and exercise capacity.
improvement in KCCQ-OS with EVOQUE TTVR at 1 year compared to baseline

of EVOQUE TTVR patients in NYHA Class I/II at 1 year

improvement in 6MWD with EVOQUE TTVR at 1 year compared to baseline


KCCQ-OS improvement

NYHA Class improvement

6MWD improvement
improvement in KCCQ-OS with EVOQUE TTVR at 1 year compared to baseline
of EVOQUE TTVR patients in NYHA Class I/II at 1 year
improvement in 6MWD with EVOQUE TTVR at 1 year compared to baseline