Advanced calcium-blocking technology*1
Pour le canadien français, veuillez suivre ce lien.
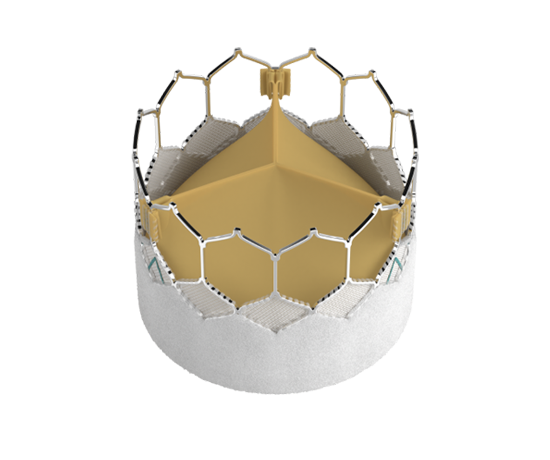

SAPIEN 3 Ultra valve, powered by RESILIA tissue

Advanced calcium-blocking technology*1
Same tissue technology used in the #1 implanted surgical valve in the Canada

Potential to improve valve longevity and reduce reintervention*†1

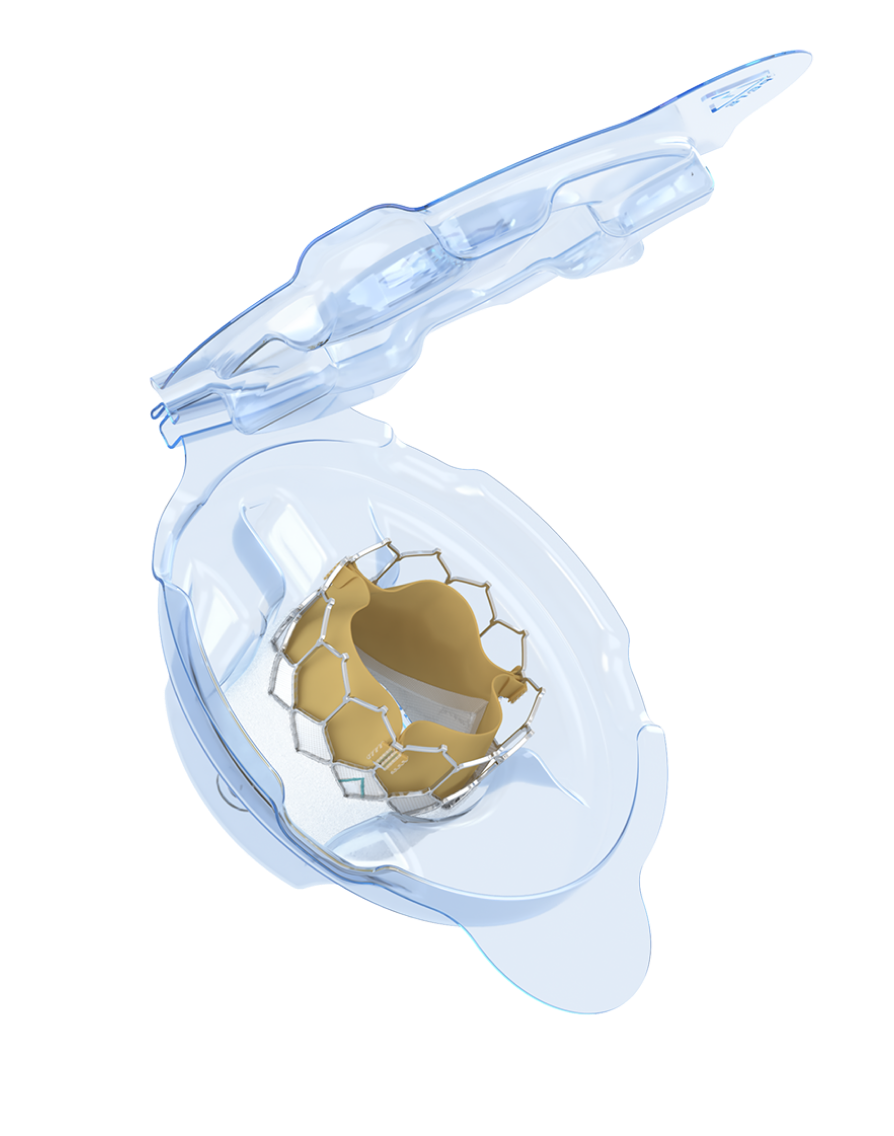
The only transcatheter heart valve (THV) with dry tissue storage
*No clinical data are available that evaluate the long-term impact of RESILIA tissue in patients. †RESILIA tissue tested against tissue from commercially available bovine pericardial from Edwards Lifesciences in a juvenile sheep model. Flameng, et al. J Thorac Cardiovasc Surg.
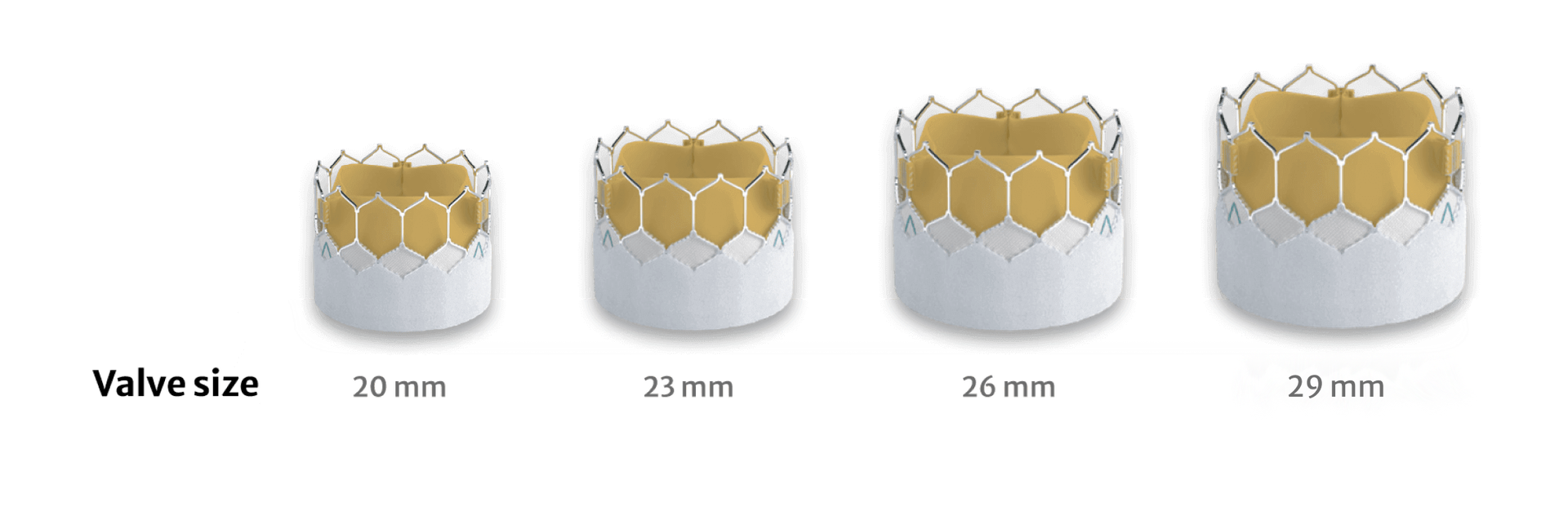
Building on the benefits of the SAPIEN 3 platform
*The PARTNER 3 Trial, SAPIEN 3 TAVR proven superior to surgery on the primary endpoint of all-cause death, all stroke, and rehospitalization (valve-related or procedure-related and including heart failure) at one year, and multiple pre-specified secondary endpoints in low risk patients.
PARTNER 3 Trial 5-Year Results in Low-Risk Patients - Low rates of cardiovascular mortality through five years (5.5% SAPIEN 3 TAVR to 5.1% SAVR). Low rates of all-cause mortality through five years (10.0% SAPIEN 3 TAVR vs. 8.2% with SAVR). Low rates of disabling stroke through five years (2.9% SAPIEN 3 TAVR to 2.7% SAVR). Low rates of stroke through five years (5.8% SAPIEN 3 TAVR vs. 6.4% SAVR). Lower rates of rehospitalization with SAPIEN 3 TAVR through five years (13.7% vs. 17.4%).
Stable-capping blocks calcium from binding to the tissue*1
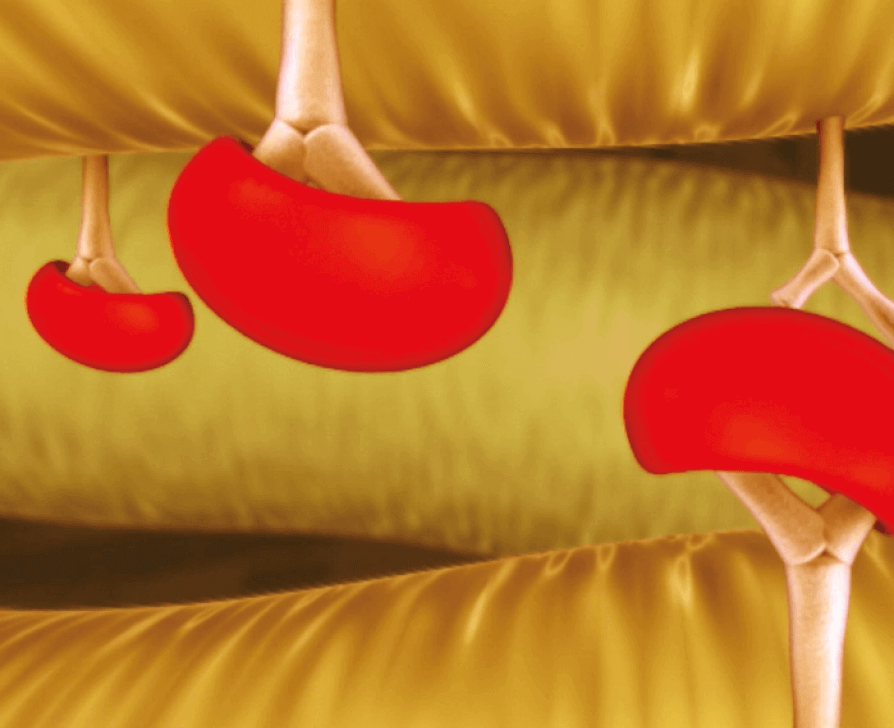
*No clinical data are available that evaluate the long-term impact of RESILIA tissue in patients

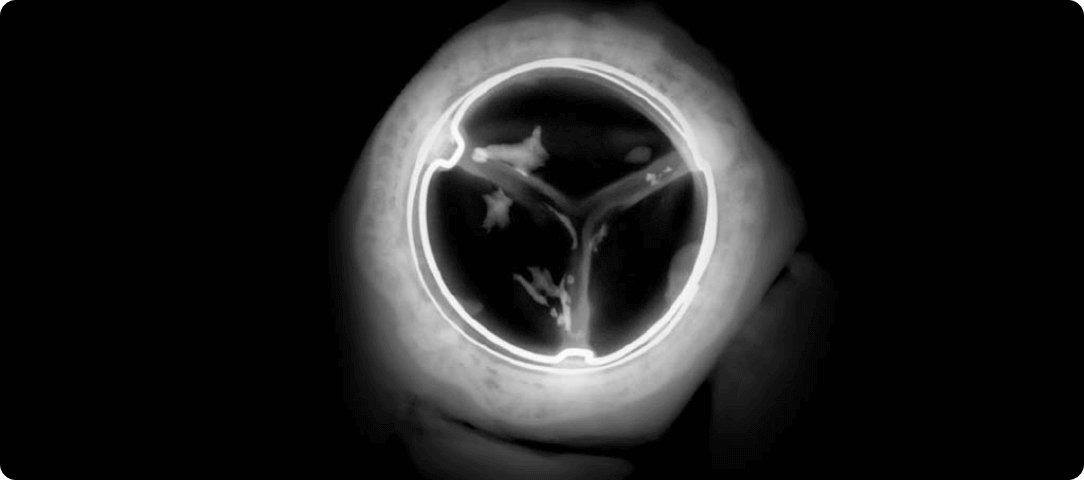
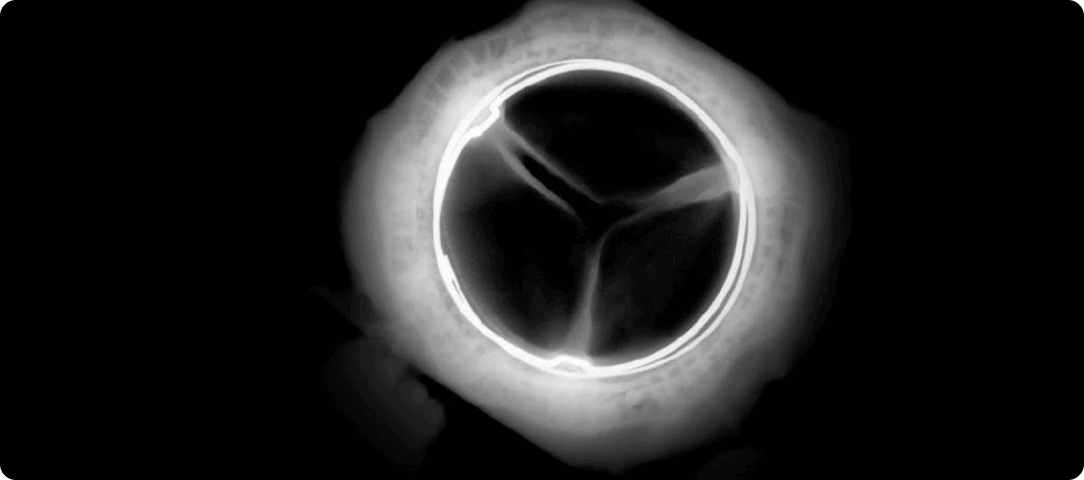
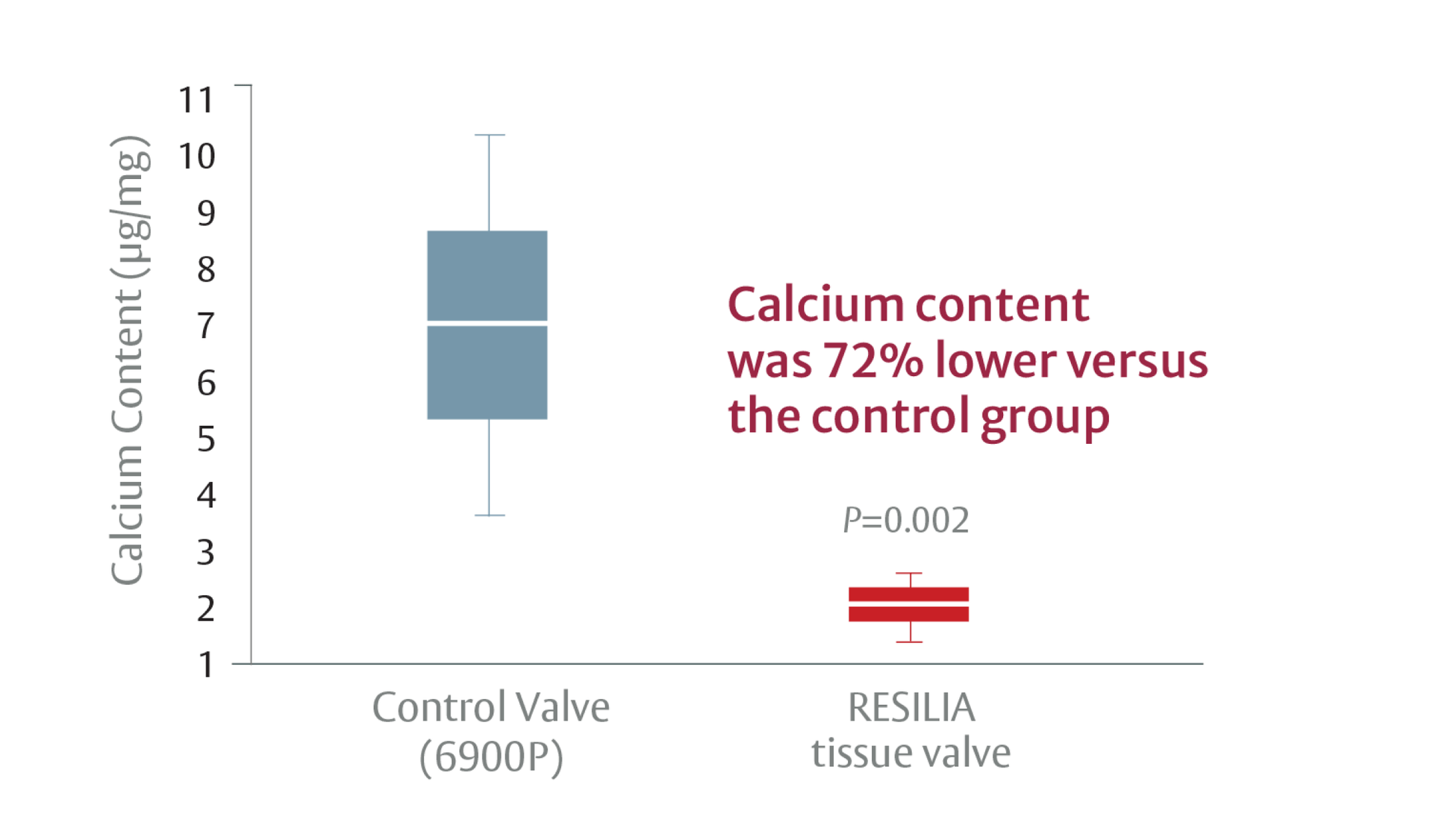
*RESILIA tissue tested against tissue from commercially available bovine pericardial valves from Edwards Lifesciences in a juvenile sheep model. Flameng, et al. J Thorac Cardiovasc Surg. 2015;149:340-345.
Read the latest clinical trial results for aortic valve replacement with RESILIA tissue