Valve platform
Proven performance of the PERIMOUNT valve design—a design with published clinical durability of over 20 years2-4
Feb 22 is Heart Valve Disease Awareness Day — Learn more about the HVD symptoms at Listen to your Heart


As the next offering in Edwards Lifesciences’ class of resilient bovine pericardial valves, ready-to-implant‡ KONECT RESILIA aortic valved conduit helps patients maintain their active lifestyles and reduces the complexity of bio-Bentall procedures.*
Edwards' integrity preservation technology transforms bovine pericardial tissue into RESILIA tissue, effectively eliminating free aldehydes, while protecting and preserving the tissue.
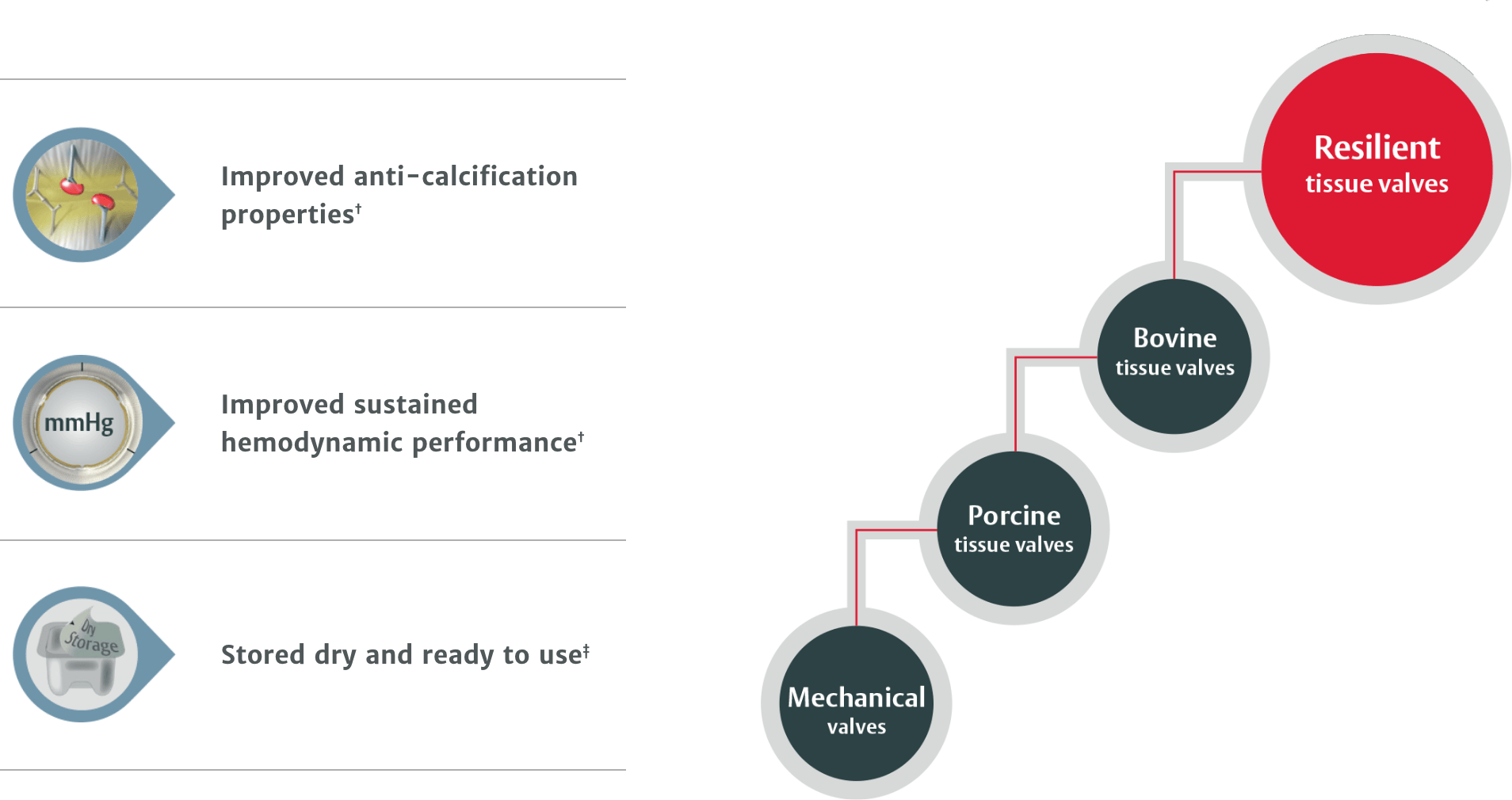
RESILIA tissue is the first to deliver the combination of:
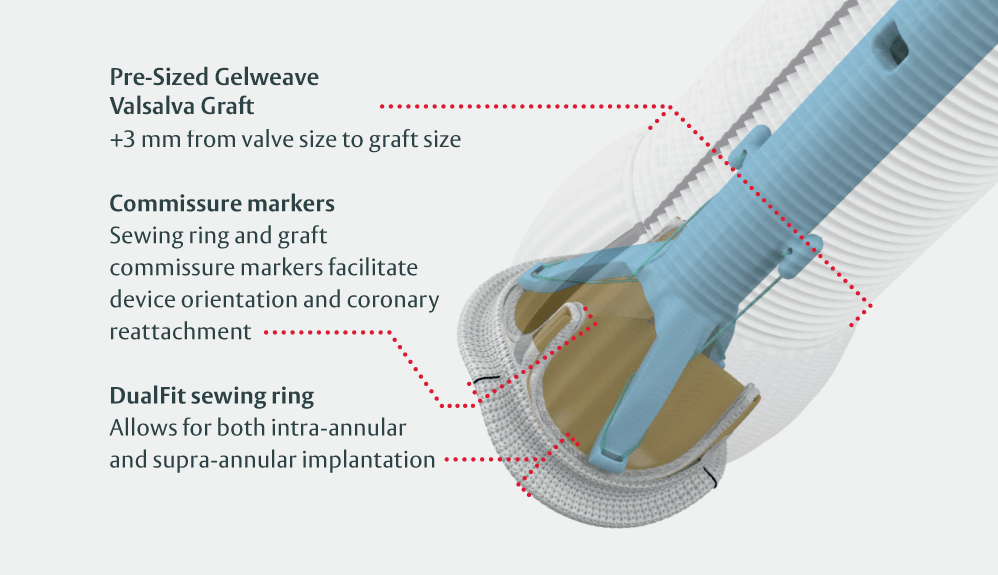
KONECT RESILIA aortic valved conduit is built on the proven performance of both the Carpentier–Edwards PERIMOUNT valve platform and the Gelweave Valsalva graft.

Proven performance of the PERIMOUNT valve design—a design with published clinical durability of over 20 years2-4

The world’s first anatomically designed aortic root graft with over 15 years of aortic root surgery clinical experience5,6
The pre-assembled KONECT RESILIA aortic valved conduit intuitively eliminates procedural steps§, which is especially important in emergency cases.

Edwards’ quality-controlled pre-assembly of valve and Gelweave Valsalva graft provides a consistent and reliable hemostatic connection

Pre-mounted to an easy-access, single-cut release holder
* By eliminating procedure steps.
† RESILIA tissue tested against commercially available bovine pericardial tissue from Edwards in a juvenile sheep model.1
No clinical data are available that evaluate the long-term impact of RESILIA tissue in patients.
‡ Consult instructions for use for device preparation instructions.
§ As compared to self-assembled tissue valved conduits.



Intended use and indications for use
The KONECT RESILIA AVC, Model 11060A, is intended for use as a replacement for the aortic heart valve and the ascending aorta.
The KONECT RESILIA AVC, Model 11060A, is indicated for patients who require replacement of their native or prosthetic aortic valve, and the associated repair or replacement of a damaged or diseased ascending aorta.
The KONECT RESILIA AVC, Model 11060A, should only be used with the dedicated sizers that replicate the DualFit sewing ring (Model 1190 Sizers and Tray).
Materials list
Tissue Platform
General Product Information
We are committed to providing your institution, clinicians and staff with the highest levels of customer service and support to ensure seamless product implementation and ongoing use, including:
24/7 Technical support
For product information and orders