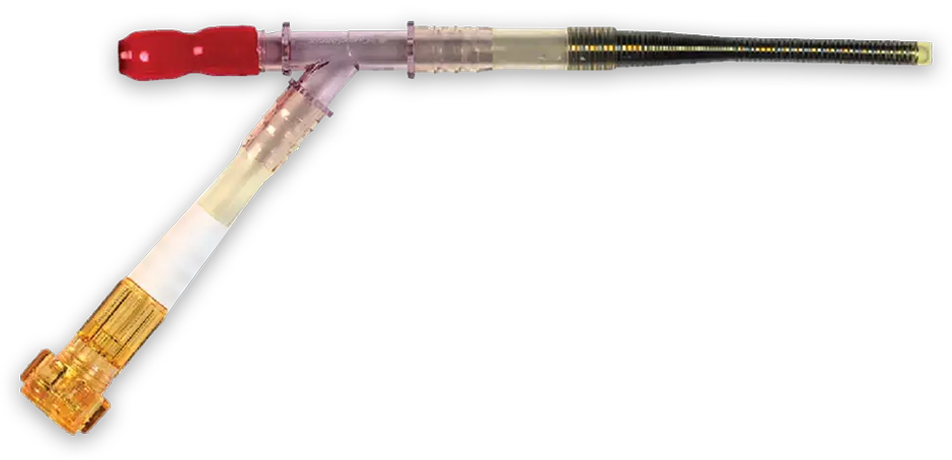
EndoReturn device
The EndoReturn arterial cannula and 19 Fr (6.3 mm) arterial cannula are indicated for patients undergoing cardiopulmonary bypass. They are intended to deliver oxygenated blood for cardiopulmonary bypass during surgery. The EndoReturn arterial cannula with hemostasis valve also allows the hemostatic introduction and removal of vascular catheters such as the EndoClamp aortic catheter.
Description:
- Kits include a wire-reinforced cannula with hemostasis valve, an introducer and a guidewire
- The cannulae have a wire-reinforced section to provide kink resistance and flexibility
- Tapered tips to aid in insertion and advancement into the femoral artery
- Hemostasis valve designed to allow passage of catheters, such as the EndoClamp aortic catheter
- The introducers accept a .038 in. (0.97 mm) guidewire and are marked to simplify assembly and indicate alignment
- A lubricious coating is applied to the surface of the cannula body, designed to ease insertion and retraction of catheters and introducers
Models & specifications
| Model | Description | Size | Image |
| ER21B | EndoReturn device | 21 Fr |  |
| ER23B | EndoReturn device | 23 Fr |  |
| IS19A | Introducer sheath | 19 Fr |  |